ADVERTISEMENT
Treatment Updates for Atopic Dermatitis
 Atopic dermatitis (AD), the most common form of eczema, is a multifaceted, chronic relapsing inflammatory skin disease with a complex pathogenesis. In the majority of cases, AD presents in infants and children, but one-third of cases persist into adulthood. In the United States, 18 million adults (7.2%) and 9.6 million (13%) of children younger than 18 years have AD, according to the National Eczema Association.
Atopic dermatitis (AD), the most common form of eczema, is a multifaceted, chronic relapsing inflammatory skin disease with a complex pathogenesis. In the majority of cases, AD presents in infants and children, but one-third of cases persist into adulthood. In the United States, 18 million adults (7.2%) and 9.6 million (13%) of children younger than 18 years have AD, according to the National Eczema Association.
In addition to a poorer health-related quality of life and social, academic, and occupational impacts, the financial burden of AD is high. A recent report on the burden of AD placed a conservative estimate of the annual costs (direct and indirect) at $5.2 billion in the Unites States.
Advances in Treatment
Until recently, treatment for AD was primarily limited to topical corticosteroids and systemic immunosupressants. Two FDA-approved therapies for AD are offering new treatment options for individuals living with skin disease.
Eucrisa
Eucrisa (crisaborole ointment, 2%; Pfizer) received FDA approval for mild to moderate AD in patients aged 2 years and older in December 2016. This topical phosphodiesterase-4 inhibitor marked the first FDA-approved drug for eczema in 15 years.
The safety and efficacy of Eucrisa were established in two identically designed, vehicle-controlled, double-blind, phase 3 studies (AD-301 and AD-302) with a total of 1522 participants with mild to moderate AD. The results published in the Journal of the American Academy of Dermatology showed patients receiving Eucrisa achieved greater response with clear or almost clear skin at day 29 of treatment. Additionally, Eucrisa demonstrated a favorable safety profile and treatment-related adverse events were mild to moderate in severity. The most common adverse event was application site pain, primarily reported as burning or stinging.
Dupixent
In March 2017, the FDA approved Dupixent (dupilumab; Sanofi/Regeneron), the first subcutaneous biologic treatment for moderate to severe AD in adults whose disease is not adequately controlled with topical prescription therapies or when those therapies are not available. It can be used with or without corticosteroids. Dupixent is a human monoclonal antibody that is designed to specifically inhibit overactive signaling of two key proteins, interleukin (IL)-4 and IL-13, which are believed to be major drivers of the persistent underlying inflammation in AD.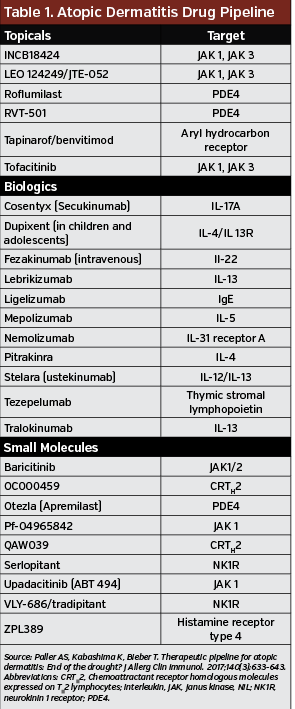
Phase 3 data from the identically designed SOLO 1 and SOLO 2 trials of 1379 participants with inadequately controlled moderate to severe AD showed that Dupixent improved the signs and symptoms of AD vs placebo after 16 weeks of treatment. The most common adverse events in both studies were exacerbations of AD, injection-site reactions, and nasopharyngitis, according to findings published in the New England Journal of Medicine.
Recently, LIBERTYAD CHRONOS evaluated the long-term safety and efficacy of Dupixent with topical corticosteroids vs placebo with topical corticosteroids in adults with moderate to severe AD. Results from the 1-year, randomized, double-blinded, placebo-controlled, phase 3 study published in Lancet showed that Dupixent added to standard topical corticosteroid treatment improved AD signs and symptoms, with acceptable safety.
Treatment Costs
These emerging therapies have the potential to change the paradigm of care, although there could be barriers to optimal care, including cost. A 60-g tube of Eucrisa is $580 before any discounts and or rebates. However, it is Dupixent that could have a significant impact on spending, with a price tag of $37,000 and a large patient population. Express Scripts reported that approximately 400,000 patients in the United States may be candidates for treatment with Dupixent, who will have to stay on the drug long term in order for continued disease relief.
In a review of the pipeline of drugs for AD published in the Journal of Allergy and Clinical Immunology, Amy S Paller, MD, MS, and colleagues wrote, “The extent of their effect on patent care will depend on their affordability and accessibility to patients with AD from a broad range of socioeconomic groups with different insurance status.”
The marketers of Eucrisa and Dupixent launched savings and support programs, which include copay cards for eligible patients who meet specific criteria.
Atopic Dermatitis Drug Pipeline
The pipeline of drugs in development for AD is growing. For example, one promising biologic agent in development is nemolizumab, an IL-13 receptor agonist the inhibits the itch pathway, which is common symptom in individuals with AD. If successful in clinical trials, it would be the first biologic therapy designed to control itch. Table 1 highlights some of new and emerging therapies in phase 2 and phase 3 development.
“The robust drug pipeline shows that manufacturers see a large potential market for atopic dermatitis treatments, but competing treatments also give payers the opportunity to implement additional utilization management tools to keep spend in line,” said Troy Brennan, MD, executive vice president, chief medical officer, CVS Health.






















