ADVERTISEMENT
Autoimmune Diseases: Psoriasis and Atopic Dermatitis
This will serve as part 2 of the 3-part First Report Managed Care Trends Report on autoimmune diseases. This second part will report the findings from survey participants on psoriasis and atopic dermatitis.
Data for this Trends Report was generated through a comprehensive survey of managed care professionals. Survey questions were developed by the editorial staff and vetted by 9 members of the Editorial Advisory Board. The types of questions asked in the survey focused on demographics, institutional practices, treatment guidelines, compliance and adherence measures, prior authorization criteria, and treatment decisions for some drug classes used in the management of autoimmune disease. The survey participants were also asked to provide comments on the challenges of treating the 5 autoimmune diseases covered in Trends Report from a managed care perspective.
The survey was distributed via a recognized survey platform and sent to the universe of managed care professionals from our email database. A total of 39 managed care professionals completed the survey with 11 partial completions. Data was then compiled based on all of the participants to generate this report.
For the survey participant demographic data, 93.9% said their organization has responsibility for commercial insurance plans. Nearly one-quarter of the survey participants (24.5%) said >5,000,000 lives are covered by their plan. Forty percent reported that their primary formulary is closed. In terms of generic dispensing rate, 32.7% said their generic dispensing rate is ≥85.0%. For complete demographic information, along with the survey findings on rheumatoid arthritis and psoriatic arthritis, please see the September issue.
The complete Trends Report will be available for download on Managed Health Care Connect at www.managedhealthcareconnect.com/supplements.
Psoriasis
Psoriasis is a chronic, genetic, immune-mediated inflammatory, multisystem skin disease affecting 7.5 million people in the United States.1,2 Five types of psoriasis exist and include plaque, guttate, inverse, pustular, and erythrodermic, of which 80% to 90% of people have plaque psoriasis. Psoriasis is generally characterized by patches of abnormal skin that are red, itchy, and scaly and most commonly manifests on the scalp, knees, elbows, hands, and feet.3 Psoriasis triggers are not universal. According to the National Psoriasis Foundation, stress, injury to skin, and reactions to medication are established psoriasis triggers.4 The economic burden of psoriasis is significant. In 2013, the direct costs associated with psoriasis ranged from $51.7 billion to $63.2 billion.5
Currently, there is no known cure for psoriasis. Treatments include traditional systemic and targeted oral agents and biologics. Several pharmacological therapies are in the drug pipeline to treat this often debilitating autoimmune disease.6,7
Survey Results
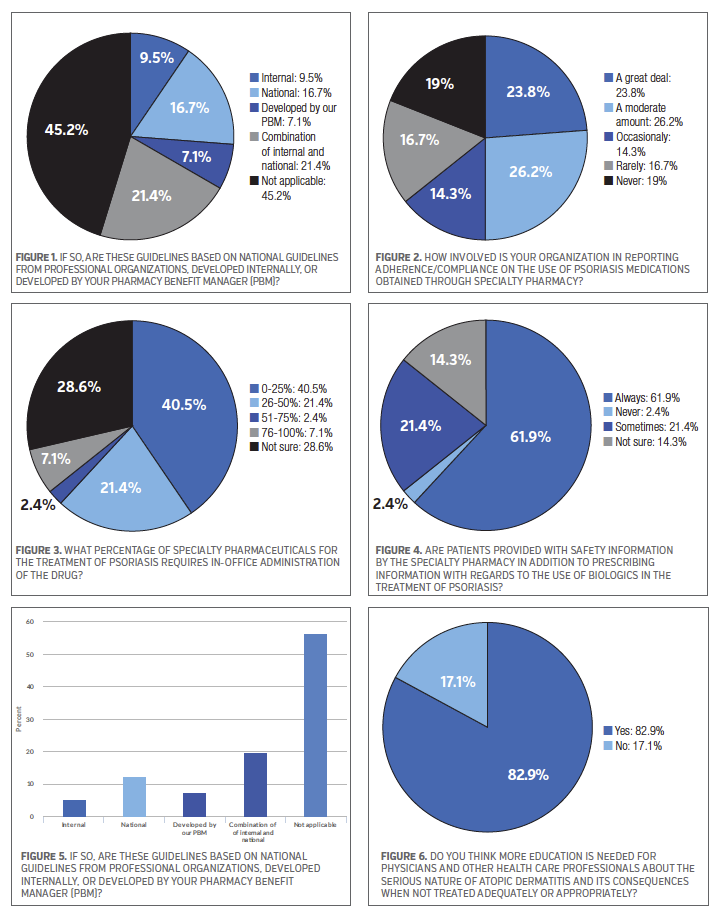
The survey participants were almost evenly divided in their response to whether their organization currently has treatment guidelines in place for the treatment of psoriasis, 48.8% said “yes” and 51.2% said “no.” In terms of the guidelines they have in place, 21.4% of the survey participants said these guidelines are based on a combination of internally- and national-developed guidelines, while 16.7% said they are based on national guidelines from professional organizations, and 7.1% said the guidelines are developed by their pharmacy benefit manager. See Figure 1.
When asked if their organization currently has prior authorization screening criteria for access to specialty pharmacy drugs to treat psoriasis, 69.0% of the survey participants said “yes.” The survey participants were next asked about their organization involvement in reporting adherence/compliance on the use of psoriasis medications obtained through a specialty pharmacy, 26.2% said “a moderate amount” and 23.8% said “a great deal.” Another 16.7% said “rarely.” See Figure 2.
Some specialty pharmaceuticals used in the treatment of psoriasis require in-office administration of the drug. Therefore, the survey participants were asked to select a percentage range that best represents their understanding of how many specialty pharmaceuticals require in-office administration to treat psoriasis, and 40.5% of the participants said 0% to 25%. Another 21.4% said 26% to 50%. However, nearly thirty percent (28.6%) of those who took the survey said they were not sure what percentage of specialty pharmaceuticals for psoriasis requires in-office administration. See Figure 3.
The majority of the survey participants (61.9%) said patients are “always” provided with safety information in addition to prescribing information with regards to the use of biologics in the treatment of psoriasis, while 21.4% said “sometimes.” See Figure 4.
Novel oral therapies, referred to as small molecule inhibitors, are emerging for the treatment of psoriasis.6 Apremilast (Otezla) is one example of a new oral small molecule therapy for psoriasis. It is a phosphodiesterase-4 inhibitor that received FDA approval8 in 2014. The survey participants were asked if they are seeing an increase in the utilization and prescribing of apremilast for psoriasis over the past 6 months, and the survey participants were split—35.7% said “yes” and 35.7% said “no.” Continuing with the topic of drug therapies, the survey participants were also split when asked if they have preferred biologic agents for the treatment of psoriasis—50.0% said “yes” and 50.0% said “no.”
Atopic Dermatitis
Atopic dermatitis is a common skin disease resulting from too many reactive inflammatory cells in the skin.9 While the disease is primarily found in children, it affects more than 30 million Americans.10 The disease is often referred to as eczema, dermatitis, atopic eczema, and atopic dermatitis. The condition presents as itchy, dry, red, scaly patches on the skin. These itchy patches generally appear on the face, forehead, and scalp, but can also be present anywhere on the body.11 Because atopic dermatitis is an uncomfortable chronic skin condition that can have a significant effect on quality of life, treatments are aimed at preventing atopic dermatitis from worsening, calming the skin, relieving the pain and itch, reducing emotional stress, and preventing infections.12,13 A treatment plan often includes emollients to keep the skin moisturized and topical steroids to bring flare ups under control, as well as lifestyle changes.
Survey Results
More than half of the survey participants (53.7%) said “yes” when asked if they consider atopic dermatitis to be an autoimmune disease based on the National Eczema Association’s statement14 that atopic dermatitis occurs at a molecular level.
When asked if their organization is actively promoting multidisciplinary management of atopic dermatitis, nearly sixty percent (58.5%) said “no,” while 19.5% said “yes.”
On the topic of treatment guidelines for atopic dermatitis, 36.6% of the survey respondents said their organization currently has treatment guidelines in place to treat this disease state. Among those survey participants who have treatment guidelines in place, 19.5% said they are based on a combination of internally- and national-developed guidelines, while 12.2% said they are based on national guidelines from professional organizations. See Figure 5.
Less than half of the survey respondents (46.3%) said “yes” when asked if their organization currently has prior authorization screening criteria for the treatment of atopic dermatitis.
A large majority of the survey respondents (82.9%) said they think more education is needed for physicians and other health care professionals about the serious nature of atopic dermatitis and its consequences when not treated adequately or appropriately. See Figure 6.
When asked if they are aware of pipeline agents for the treatment of atopic dermatitis, the majority of survey respondents (68.3%) said “no” compared with 31.7% who said “yes.”
Conclusion
The symptoms associated with psoriasis and atopic dermatitis can have a significant impact on quality of life for those living with these chronic skin diseases. Often individuals with these autoimmune diseases require lifelong management. Yet, the majority of survey respondents reported that their organizations do not have treatment guidelines in place to treat psoriasis and atopic dermatitis (51.2% and 63.4%, respectively). Because some specialty drugs are used in the management of psoriasis and atopic dermatitis, prior authorization screening criteria for access to these drugs has become a key issue among managed care stakeholders. The majority of the survey participants reported that their organization requires this step for psoriasis (69.0%). However, more than half of those surveyed said this process is not done for atopic dermatitis (53.7%).
Multidisciplinary management of atopic dermatitis is recommended, however, 58.5% of the survey respondents said their organization is not actively promoting it. Education is an important component is the management of atopic dermatitis. The majority of participants (82.9%) agreed that more education is needed for health care professionals about the serious nature of this disease state and the consequences when it is not treated adequately or appropriately. This was echoed by some of the survey respondents when they were asked to share their general thoughts and concerns for treating atopic dermatitis through an open-ended section of the survey (Box). Although medication adherence continues to be a challenge in managing psoriasis and atopic dermatitis, lack of treatment efficacy and drug cost play an important role.
REFERENCES
1. Menter A, Gottlieb A, Feldman, SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826-850.
2. National Psoriasis Foundation. The Psoriasis and Psoriatic Arthritis Pocket Guide: Treatment Options and Patient Management. 3rd ed. Portland, OR; 2009.
3. About psoriasis. National Psoriasis Foundation website. www.psoriasis.org/about-psoriasis. Accessed September 9, 2016.
4. Psoriasis causes and triggers. National Psoriasis Foundation website. www.psoriasis.org/about-psoriasis/causes. Accessed September 9, 2016.
5. Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: A systematic review. JAMA Dermatol. 2015;151(6):651-658.
6. Yiu ZZ, Warren RB. Novel oral therapies for psoriasis and psoriatic arthritis. Am J Clin Dermatol. 2016;17(3):191-200.
7. Drug pipeline. National Psoriasis Foundation website. https://services.psoriasis.org/drug-pipeline/index.php. Accessed June 10, 2016.
8. Oral OTEZLA® (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis [news release]. Summit, NJ: Celgene Corporation; September 23, 2014. https://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed September 9, 2016.
9. Causes of atopic dermatitis. National Eczema Association website. https://nationaleczema.org/atopic-dermatitis-in-children/atopic-dermatitis/. Accessed September 9, 2016.
10. What is eczema? National Eczema Association website. https://nationaleczema.org/eczema/. Accessed September 9, 2016.
11. Atopic dermatitis: signs and symptoms. American Academy of Dermatology website. https://www.aad.org/public/diseases/eczema/atopic-dermatitis#symptoms. Accessed September 9, 2016.
12. Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: The National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152(8):873-874.
13. Atopic dermatitis: diagnosis and treatment. American Academy of Dermatology website. https://www.aad.org/public/diseases/eczema/atopic-dermatitis#treatment. Accessed September 9, 2016.
14. Atopic dermatitis found to be an autoimmune disease. National Eczema Association website. https://www.nationaleczema.org. Accessed September 9, 2016.




















