How to Select an Extracellular Matrix for Wound Repair: A Comprehensive Review
© 2023 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. An extracellular matrix (ECM) is a network of proteins and other molecules that provide support and structure to cells and tissues in the body. Since its discovery in 1930, researchers have reproduced the ECM through an array of evolving technologies, developing products that accelerate healing times, minimize scarring, and reduce pain. When selecting which ECM product to use, physicians rely on personal experience while considering wound location, type of tissue lost, exposed structures, chronicity, and even the patient’s religious preferences. While comparison trials between a few different types of ECMs exist, there lacks a thorough investigation that assesses a majority of ECMs against each other.
Methods. Herein, we conducted a literature review using the PubMed database and utilized 71 articles to identify the best ECM for wound healing and positive patient outcomes. The primary search terms included extracellular matrix, xenograft, porcine, bovine, allograft, bioengineered matrix, acellularized fish skin, wounds, wound healing, and wound care. We did not exclude any specific type of research, but predominantly reviewed clinical trials, case series, and other review articles. We focused on the most popular and commonly used ECMs and constructed our results into the Table.
Results. We compared the indications, advantages, and disadvantages of each ECM and concisely illustrated these findings to provide a guide on how to select an ECM (Table). Allografts, whether they are glycerol or cryopreserved, suffice as a treatment choice and are superior to exposure healing. However, they do not produce healing at the same rate or quality as bioengineered matrices, porcine and bovine xenografts, or acellularized fish skin (AFS). Bioengineered matrices and porcine and bovine xenografts offer antimicrobial properties, low immunogenicity, cost effectiveness, and availability. The compromise with these ECMs is with healing times and cosmesis. Acellularized fish skin (AFS) provides diverse utility, antimicrobial activity, low immunogenicity, faster healing times, and cosmetic superiority. However, AFS yields a potential cost burden and is not plentiful or easily accessible in some parts of the world.
Conclusions. Our findings assist in removing the subjectivity component of selecting an ECM and suggest further comparison or head-to-head trials would yield a more algorithmic approach to wound healing. We suggest to consider implementing the Disabilities of the Arm, Shoulder, and Hand (DASH) score as an additional objective comparison method in these future trials.
Introduction
An extracellular matrix (ECM) is a network of proteins and other molecules that provide support and structure to cells and tissues in the body. In 1930, a French team of scientists led by Jean Nageotte synthesized the first known ECM by solubilizing and reconstituting collagen fibrils.1,2 Since the initial discovery, researchers have reproduced the chemical, physical, and biological properties of the ECM through an array of evolving technologies, developing products that accelerate healing times, minimize scarring, and reduce pain. When selecting which ECM product to use, physicians rely on their own experience while considering wound location, type of tissue lost, exposed structures, chronicity, and even the patient’s religious preferences. While comparison trials between a few different types of ECMs exist, there lacks a thorough investigation that assesses a majority of ECMs against each other.

Methods and Materials
Herein, we conducted a literature review using the PubMed database and utilized 71 articles to identify the best ECM for wound healing and positive patient outcomes. The primary search terms included extracellular matrix, xenograft, porcine, bovine, allograft, bioengineered matrix, acellularized fish skin, wounds, wound healing, and wound care. We did not exclude any specific type of research, but predominantly reviewed clinical trials, case series, and other review articles. Due to the limited available data, we did not tightly constrain our study selection nor the date range of publications, although a majority of the articles evaluated were published after the year 2000. We focused on the most popular and commonly used ECMs and knowingly omitted autografts since they presented a unique set of additional variables that diverged from the scope of this paper. We then summarized our comparisons of each ECM in the Table.
Results
Allografts
Indication for a skin allograft typically occurs when autografts are unavailable. They are almost always taken from cadavers because living donor tissue offers minimal healing advantages to justify the need for pre-harvest investigations, anesthesia, surgery, pain, wound healing issues, scarring, and hospitalization.3 Once screening of the cadaver tissue is complete and proper authorization has been obtained, the skin is procured, processed, and preserved for transportation.
Cryopreservation and glycerol preservation are the 2 primary techniques for preparing allografts. Both methods maintain structural integrity and produce low pain levels similar to autografts.4,5 However, between the 2, cryopreservation yields a higher level of tissue viability (n = 48, P < .05) while glycerol preservation allows for longer storage times and is more cost effective.4,6 In a comparison study simulating clinical conditions on a porcine wound model, the 2 techniques elicited equal take rates after week 1.6 Fibroblast hyperplasia and neovascularization rates were also not found to be statistically significantly different between the 2 methods.6 However, the cryopreservation group produced more microscopic inflammation after week 2, and at week 5, glycerol preservation allografts yielded higher rates of collagen fiber synthesis (2.67 ± 0.47 vs 2.33 ± 0.47).6 Therefore, this suggests allografts undergoing glycerol preservation are not impeded by microscopic inflammation and heal quicker than cryopreserved allografts. Nevertheless, regardless of healing rates, final clinical outcomes between these 2 techniques of preparing skin allografts are not significantly different.4,6
When comparing cryopreserved allografts to healing by natural exposure, a difference in clinical outcomes becomes evident. Leicht et al demonstrated that 15 out of 25 patients (60%) receiving cryopreserved allografts healed at 14 days compared with 7 out of 23 patients (30.4%) healing by natural exposure.7 They also identified that the cryopreserved allografts tended to yield better cosmetic scores than those of the exposure group, but the difference was not statistically significant (n = 48, P = .11).7
Allograft Discussion and Evaluation
Assessing allografts overall as a type of ECM, we deduce that they are superior to exposure healing, but neither glycerol-preserved allografts nor cryopreserved allografts exhibit a definitive advantage over one another. However, both types of allografts possess the desirable properties of autologous skin, such as reducing pain, adhering to the wound bed, preventing wound desiccation, stimulating vascularization, improving thermoregulation, protecting against bacterial contamination, providing a dermal matrix, and creating a wound closure that decreases water, electrolyte, and protein loss.8 Therefore, we find allografts to be a satisfactory ECM and most beneficial for limiting pain when used as a prelude to an autograft.
Bioengineered Matrices
Another type of ECM we reviewed is a bioengineered matrix. Bioengineered matrices are synthesized from a variety of materials and allow for the transmission of water vapor while providing several benefits, including rapid and sustained wound adherence, impermeability to external bacteria, and pliability.9 These nonantigenic ECMs promote cell migration, proliferation, and growth. Low in cost with an indefinite shelf life and minimal storage requirements, bioengineered matrices have become a popular choice of ECM in the wound healing community.9
Composition
Silicone and nylon. One type of bioengineered matrix we reviewed consisted of a silicone membrane and nylon mesh impregnated with porcine dermal collagen. This bioengineered matrix was compared with frozen human cadaver allografts in a 2-year multicenter study.10 The number of dressing changes, amount of area changed, purulence, graft take, final results, and number of complications for each matrix were analyzed. For each of these variables, no significant difference was found between sites covered with allografts and sites covered with this porcine-impregnated silicone membrane and nylon mesh extracellular matrix.10 A later comparison trial then revealed the superiority of this type of bioengineered matrix to the cadaveric allograft in both procedure time (cadaveric allograft, 54.78 ± 74.59 minutes vs porcine-impregnated silicone membrane and nylon mesh matrix, 21.12 ± 10.66 minutes; n = 45, P = .022) and associated cost per percent total body surface area (cadaveric allograft, $301.03 ± $141.42 vs porcine-impregnated silicone membrane and nylon mesh matrix, $166.09 ± $80.24; n = 45, P = .0003).11 Therefore, this demonstrated a bioengineered matrix could be as effective in wound coverage as frozen human cadaveric allografts while also being faster to apply and less expensive.
Foreskin-derived ECMs. In a different 2-year clinical trial examining multiple elements of wound healing outcomes, patients treated with a bioengineered matrix consisting of living foreskin-derived keratinocytes, fibroblasts, and bovine collagen were compared with those of a control group of patients receiving autografts and/or autografts meshed with allografts.12 Patients in the foreskin-derived bioengineered matrix group achieved significantly higher rates of normal pigmentation (45% vs 13%; n = 38, P = .0005), vascularity (47% vs 16%; n = 38, P = .0005), pliability (61% vs 13%; n = 38, P < .0001), and scar height (61% vs 37%; n = 38, P = .0117) than were seen in the control group.12 In addition, 58% of patients with wounds treated with this foreskin-derived bioengineered matrix achieved an overall cosmetic appearance that was better than that seen in the control group; another 26% of patients had results that were equivalent to those of the control group (n = 38, P = .0037).12 Consequently, this trial provided evidence supporting the cosmetic superiority of bioengineered matrices over autografts and allografts.
This cosmetic superiority was also replicated when this same foreskin-derived bioengineered matrix was compared with secondary intention healing.13 Furthermore, a similar bioengineered ECM made of neonatal dermal fibroblasts without the bovine collagen demonstrated faster closure (n = 25, P = .056) and higher rates of both complete closure (50% vs 8% n = 25, P = .03) and 50% closure (75% vs 23%; n = 25, P = .018) within 8 weeks compared with conventional wound closure.14 These results illustrated that the improved cosmesis of bioengineered matrices is even accomplished at faster rates.
Polyurethane dermal matrix. One other bioengineered ECM worth mentioning is derived from a man-made synthetic polyurethane dermal matrix. Mechanistically, it creates suitable wound beds for skin grafts by promoting the infiltration of cellular materials and supporting dermal reconstruction. Application occurs in a 2-step process where the biodegradable temporizing matrix (BTM) is first laid on a clean wound bed, and then a split-skin graft is applied weeks later. In the first phase, the BTM allows cells and blood vessels to form a vascularized neo-dermis in as little as 2 weeks.15 Once the polyurethane matrix has degraded naturally via hydrolysis, the sealing membrane of the product may be removed, and a split-thickness skin graft can be applied.15 In vitro studies have confirmed BTM to be biocompatible.16 This type of bioengineered ECM is indicated for use in managing partial- and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic and vascular ulcers, surgical wounds, and trauma wounds, including deep second-degree burns, third-degree burns, and draining wounds.17 The application of this product is contraindicated in wounds with necrotic tissue present. Such wounds must be surgically debrided with the addition of antimicrobial dressings before this product is used.
Two case series highlight the benefits of this polyurethane-derived matrix. Li et al identified 33 of 35 wounds as having 100% integration of the matrix by the time the sealing membrane was removed, while 7 of the wounds that had partial graft loss were healed by secondary intention.16 In addition, 2 of the wounds had complete re-epithelization with the matrix alone and did not require split-thickness skin grafts.16 Solanki et al pointed out that the polyurethane matrix integrated, on average, 2 to 4 weeks after application and demonstrated improved concavity and a high take rate if the neo-dermis formed well.18 Therefore, one can reasonably conclude that a polyurethane dermal matrix may be a great choice for an ECM since it produces a naturally thick dermis with minimal contractures, limited tethering of underlying structures, and lower immune rejection and infection rates.16 However, more studies are needed to compare it directly with other dermal matrices.
Healing Process
Further studies also assessed advantages and disadvantages in the healing process with using bioengineered matrices. In Jones and Nelson’s review of 15 randomized control trials that used skin grafts to treat venous leg ulcers, the trials comparing bilayer artificial skin to standard of care dressings reported a significantly higher proportion of ulcers that healed with engineered, artificial skin.19 However, a prospective cohort study comparing a bioengineered matrix made of silicone with a bilaminate sheet of cross-linked bovine tendon collagen and shark glycosaminoglycans (chondroitin-6-sulfate) to decellularized dermis and allografts yielded somewhat conflicting findings.20 Compared with the bioengineered matrix, the decellularized dermis and allografts resulted in less fibrosis (n = 50, P = .047 and .03, respectively) and a larger portion of regenerated dermis (n = 50, P = .003 and < .0001, respectively).20 Therefore, these findings suggest that bioengineered matrices may be cosmetically superior but may not yield the most desirable healing outcome since the scar may be more fibrotic and regenerate less dermis.
These fibrotic scarring outcomes are further supported by a study that used a cutometer to measure skin elasticity in wounds. Glycerol-preserved allografts were found to possess significantly superior skin elasticity when compared with engineered carboxymethylcellulose-based grafts (n = 80, P = .010).21 Nevertheless, even with more fibrotic scars, bioengineered matrices still remain a viable option as an ECM.
Cosmetic and Overall Outcomes
Another important factor to consider is that cosmetic outcomes and healing properties can also vary between the different types of bioengineered matrices. Although there is limited statistically significant evidence to support this claim, there exists clear descriptive analysis demonstrating this phenomenon. For example, multiple studies suggest a bioengineered matrix made from an esterified form of hyaluronic acid to be preferred when faster wound healing is required, while a matrix made from silicone with a bilaminate sheet of cross-linked bovine tendon collagen and shark glycosaminoglycans (chondroitin-6-sulfate) is preferred when the goal is a better cosmetic outcome.22 The hyaluronic acid–derived matrix achieved re-epithelization in 83% of ulcers in a median time of 16 days, which was supported by observations of better cell regulation, extracellular matrix regeneration, and neoangiogenesis.22-24 In comparison, the silicone cross-linked bovine tendon collagen and shark glycosaminoglycans (chondroitin-6-sulfate) matrix achieved cosmetic favor due to observations of better skin color; more elastic regenerated dermis; and better overall physical, mechanical, and optical properties.22,24
In addition to cosmesis and rate of healing, preference also exists based on the type of wound being treated. For example, bioengineered matrices made of neonatal dermal fibroblasts without bovine collagen and bioengineered matrices made of living foreskin-derived keratinocytes, fibroblasts, and bovine collagen are preferred for the treatment of diabetic foot ulcers (DFUs). This does not indicate that other bioengineered matrices will not be effective but rather that multiple studies observed that these 2 types of matrices interact the best with the healing conditions of DFUs.25-29 These minor discrepancies are not meant to detract from the benefits of bioengineered matrices; instead, these reports should be used as supplemental information to help select the ECM best suited to achieve the desired patient outcome.
Bioengineered Matrices Discussion and Evaluation
Evaluating bioengineered matrices as a collective type of ECM, we acknowledge these products have the clear benefits of always being available in any quantity and not needing graft harvesting or harvest site healing. Consequently, these products carry a negligible cross-infection risk while offering cosmetic superiority, cost effectiveness, increased healing rates, faster healing rates, quicker procedure times, less pain, and fewer complications than skin grafting. However, we also understand that a fast healing and aesthetic wound does not necessarily guarantee excellent vascularization or a strong, elastic scar. Furthermore, we appreciate that bioengineered matrices produce a range of outcomes due to the variety of materials used to create them. Therefore, we recommend tailoring bioengineered matrix selection to the desired outcome. For example, a porcine-impregnated silicone membrane and nylon mesh bioengineered matrix may be a great ECM choice in military and mass casualty settings due to its short procedure time. Meanwhile, a bioengineered matrix containing living foreskin-derived keratinocytes, fibroblasts, and bovine collagen may be a great option to treat a DFU or for those wanting to optimize cosmesis and avoid psychological stress and reoperation. This breadth of utility highlights why we find bioengineered matrices to be an excellent ECM option for wound healing.
Xenograft ECMs
Looking beyond bioengineered matrices, we chose to assess xenografts as a distinct type of ECM. Xenografts are grafts obtained from animal species and placed onto the human body. These species have historically included chickens, rats, pigeons, cats, dogs, frogs, cows, and pigs.30 When xenografts are utilized as temporary wound barriers, the interaction between the graft and wound leads to a new immune response. This immune response initiates a wound healing process by stimulating cellular elements and components, such as growth and chemotactic healing factors. The newly initiated immune response results in tissue repair and re-epithelization beneath the xenograft. Capillaries and vascular structures do not proliferate into the graft and eventually the xenograft is ejected, not rejected, as new dermis forms below.31,32 Over time porcine skin became the xenograft of choice due to its histological structure similarities to human dermis.30 Consequently, a majority of research evaluates porcine as the primary xenograft and therefore will be our initial focus before elaborating on additional types of xenografts.
Porcine Grafts
Currently, porcine grafts treat partial thickness burns,33 split-thickness skin graft donor sites,34,35 and exfoliative skin conditions, such as toxic epidermal necrolysis.36 The grafts have demonstrated advantages in reducing pain, maintaining proper moisture conditions, protecting the wound from external conditions, and preventing infections due to their antibacterial nature.37 Compared with the numerous other ECMs available, porcine xenografts are relatively cost effective and affordable. In addition, there is an abundance of supply, and they provide large dressing sizes that are used for large surface areas difficult to cover with other ECMs. Porcine xenografts also can be formulated into a powder, enabling conformation to the tunneling and undermining in wounds.
Initially, porcine skin grafts were not viable options due to hyperacute rejection by the host as a result of preformed antibodies, most significantly to the α-1,3-galactose (Gal) moiety present on swine cell membranes.38 However, genetic modification of swine has allowed researchers to knock out the gene GalT-KO, which encodes α-1,3-galactosyltransferase.39 Today, acute rejection due to preformed host antibodies is rare, and there is usually no major immune response and a negligible detection of anti-donor antibodies to the graft.40-42 In addition to genetic modification, porcine skin grafts are often further prepared and preserved with glutaraldehyde, a protein with cross-linking properties. This preparation produces increased durability and efficacy of the grafts.43,44
One notable xenograft we investigated contained an extracellular collagen-rich matrix derived from porcine small intestinal submucosa (SIS). This biocompatible, acellular, nonimmunogenic, and biodegradable tri-layer of SIS had a thickness of 0.30 mm.45 Approximately 90% of this matrix was composed of collagen while ECM proteins, including glycoproteins and glycosaminoglycan, made up the remainder of the matrix. Fibronectin, one of the important glycoproteins in this matrix, allows for the attachment of many cell types, especially endothelial cells. This attachment promotes the development of connective tissue cells.46 Therefore, when this specific xenograft was placed on wounds, it acted as a native tissue scaffold, providing structural support while minimizing the inflammatory response.47 This ECM product was easily absorbed and not difficult to handle.
SIS-derived matrices have also shown great results in tissue remodeling when applied to multiple animal models. A case series by Aboulssa et al tested the clinical outcome of SIS when applied to 6 different wound types: chronic venous ulcer, nonhealing Achilles tendon vasculitic wound, Marjolin’s ulcer, posttraumatic wound, stage IV sacral-coccygeal pressure wound, and a complicated transmetatarsal amputation of gangrenous DFU of the left forefoot. The number of treatments ranged from 1 to 12 applications over a 4- to 16-week period. With the addition of the SIS, the wounds healed within the expected time periods without complications, and, in some cases, the healing process was even accelerated; this accelerated healing was thought to be due to the disruption of the prolonged inflammatory phase.48 These findings led the researchers to conclude that, in these 6 specific wound types, the standard of care is insufficient and can be improved by SIS-derived xenografts.49
Xenografts are also effective in the debridement of infected burns and ulcers. Porcine skin specifically demonstrated a reduction in heavy bacterial colonization in wound bedsand removed slough from burns and ulcers.31,32,50 Although these properties are well explored, the mechanism remains unclear. It is believed that the tight adherence of the porcine grafts is the source of antibacterial action. Furthermore, these grafts maintain proper hydration of the wound and prevent extremes of fluid collection or desiccation that often leads to necrotic and infected tissue.37 Limiting risks for infection consequently has contributed to porcine xenografts being associated with significant pain reduction.37
Bovine Xenografts
In addition to porcine xenografts, research has also explored bovine grafts, especially in the treatment of DFUs. Fetal bovine dermal repair scaffold, also known as fetal bovine collagen (FBC), is an acellular dermal matrix that is derived from fetal bovine dermis. It acts by attracting and binding the patient’s own cells and growth factors, such as vascular endothelial growth factors.51-53 This supports the revascularization and epithelization processes. Fetal dermis also contains a higher percentage of type III collagen compared with adult dermis, and this specific collagen is more active in healing and developing tissues.54 Kavros et al conducted a multicenter, prospective study of 46 patients with DFUs that had been present for a mean 286 days with an average size of 4.34 cm.2 After a single application, 59.1% of the DFUs healed within 12 weeks; with 2 applications of the FBC, an additional 22.9% healed. For the individuals whose wounds did not heal, the average wound area reduction was 71.4%.55
In another clinical trial, 207 patients with non-healing DFUs were treated with either the standard of care or FBC. In this study, Lantis et al found the mean time to closure was 43 days for FBC and 57 days for standard of care. In addition, adverse events were similar between the 2 groups. It was concluded that the addition of FBC to the standard of care offered a safer and more effective treatment than the standard of care alone.56 The results of FBC are promising, and this xenograft should be considered as a possible first line treatment for DFUs.
Xenograft Discussion and Evaluation
Xenografts have been reliable ECMs used to treat nonhealing partial-thickness burns, split-thickness skin graft donor sites, and DFUs for many years. Their ability to reduce pain, maintain ideal moisture conditions, create a wound barrier, and prevent bacterial growth have made them advantageous over other traditional treatments. Compared with other ECMs, porcine and bovine xenografts are readily available in various sizes and are relatively affordable for most patients. Therefore, we believe that xenografts are a great option for ECM replacement therapies.
Acellularized Fish Skin
In recent years, acellularized fish skin (AFS) sprouted as its own category within xenografts and has become a major rival to porcine skin grafts. AFS is primarily harvested from 2 species of fish: Nile tilapia and North Atlantic cod. The Nile tilapia, provided by fish farmers in East Africa, is currently exclusive to the tropical and subtropical regions. On the other hand, the North Atlantic cod is harvested in Iceland and available worldwide.57 The fish skin is prepared through osmotic manipulation using a light detergent, unlike other xenografts that require preparation with costly chemicals and stronger detergents.58 This allows the fish skin to maintain a strong scaffold structure without losing its important molecular components. In addition, a morcellated form of fish skin grafts exists, permitting more precise graft placement and treatment of more anatomically challenging areas. Currently, fish skin indications include treatment of partial- and full-thickness burns, pressure ulcers, chronic vascular ulcers, DFUs, trauma wounds, surgical wounds, and draining wounds.
For burn management, studies highlighted the effect of AFS on re-epithelization time, potential pain reduction, and treatment-related costs. With standard treatments, such as silver sulfadiazine cream, re-epithelization may take up to 2 weeks for superficial partial-thickness burns and greater than 3 weeks for deep partial-thickness burns.59 Júnior et al found that use of Nile tilapia grafts decreased the time until complete re-epithelialization by 1.43 days in outpatients and 1.14 days in inpatients.60 In another case-control study, Wallner et al found a combination of North Atlantic cod and enzymatic debridement to have a re-epithelization time of 22 ± 6.3 days compared with 45.6 ± 6.6 days in patients treated with an alloplastic wound substitute and enzymatic debridement. They also identified that North Atlantic cod re-epithelializes faster than split-thickness skin grafting (34.7 ± 12.5 days).61 In regard to pain intensity, both studies revealed decreased anesthetics usage when either Nile tilapia or North Atlantic cod grafts were used.60,61
When used to treat chronic, nonhealing wounds, such as vascular and diabetic ulcers, AFS grafts have also been found to provide notable benefits. Michael et al analyzed the efficacy of AFS in full-thickness DFUs by measuring the surface area of the wound beds over a 16-week period. They discovered AFS grafts decreased the mean wound surface areas by 87.57% and that 35 of 58 wounds (60.34%) fully healed within the 16 weeks.62 In another study, Yang et al conducted a prospective clinical study analyzing the role of AFS grafts in chronic diabetic, arterial, and venous ulcers in 18 patients. At 5 weeks after application of the grafts, they found a 40% decrease in mean wound surface area (n = 18, P < .05) as well as a 48% decrease in wound depth (n = 18, P < .05). Furthermore, they found 3 of the 18 patients with chronic wounds to be completely healed by the end of the 5-week period.63 These results demonstrate how AFS grafts positively affect healing and provide evidence as to why AFS grafts warrant continued investigation.
Other advantages to fish skin grafts include their ability to provide antimicrobial environments to the wounds due to the presence of omega-3 polyunsaturated fatty acids, including docosahexaenoic acid and eicosapentaenoic acid.64 In addition, fish skin contains collagen, fibrin, proteoglycans, glycosaminoglycans, and growth factors, all important components that aid in accelerated healing rates compared with standard of care options.57,65 These components also do not induce hypersensitivity reactions or lead to additional inflammatory reactions.66
Cost and Availability
It is important to note that approximately 85% of burns occur in low- and middle-income countries. Therefore, affordable burn care should be a priority and unfortunately is often overlooked.67 Compared with other xenografts, AFS grafts are more cost effective since they require less processing and a shorter manufacturing period.55 In fact, this was identified in a recent study that found patients who received treatment with Nile tilapia AFS had a reduction of up to 42% in total costs compared with standard treatment options.65,66 Although this finding is promising, we must be mindful that the Nile tilapia is currently only available in tropical and subtropical regions and that this cost benefit can only be applied to these patient populations. Data regarding the cost effectiveness of North Atlantic cod is minimal, but it should be noted that this is an expensive matrix, and additional studies evaluating its role in the cost of wound management is important.
Acellularized Fish Skin Discussion and Evaluation
Fish skin grafts have emerged as novel treatments for various wounds, such as burns and chronic ulcers. Although additional clinical trials are required before these products replace standard treatments, AFS proves to be a strong alternative due to its ability to accelerate wound healing through the presence of natural omega-3 polyunsaturated fatty acids and its ability to provide antimicrobial environments.68 In addition, although Nile tilapia AFS has regional limitations, the availability of North Atlantic cod AFS ensures that global distribution of AFS will not be an issue. However, we acknowledge that the cost burden between these 2 AFS products are not equal. Therefore, when evaluating AFS as an ECM, we believe it stands out among other treatments with its ability to reduce pain, provide improved re-epithelialization, and decrease wound depth, but the patient’s financial status should be carefully considered in conjunction AFS availability before selecting it as treatment.
Limitations
A notable limitation of our literature review is that although we were able to evaluate the different ECMs across different types of wounds, several studies primarily focused on burn wounds. The healing process should have limited variation between types of wounds, but nonetheless we acknowledge this as a potential bias. Another limitation is that we did not assess every type of bioengineered matrix or xenograft but instead focused on the most popular and commonly used ones. Therefore, we could potentially unknowingly neglect highlighting unique healing benefits of a specific type of ECM. Lastly, we did not explore cell-signaling products or placental-based therapies involving stem cells and protein stimulation of native cell receptors as they are out of the scope of this article. However, we acknowledge that although they are not ECMs per se, these treatments contribute to wound healing and a future paper exploring their applications would be valuable to the literature.
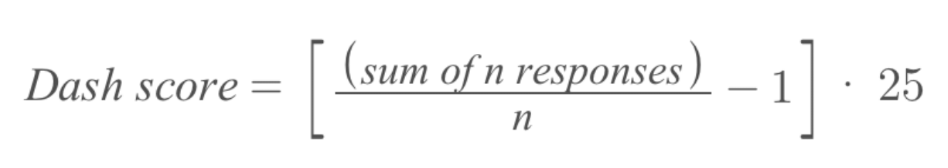
Implementation of DASH Score
Outside of these limitations, we would also like to highlight a potential novel method of assessing and comparing the efficacy of various ECM products in the future. We propose implementing the Disabilities of the Arm, Shoulder, and Hand (DASH) score. The DASH questionnaire is a self-report questionnaire that explores the ability of a patient to perform certain upper extremity activities, including “make a bed” or “use a knife to cut food.” Patients rate their abilities to perform these tasks on a 5-point scale representing no difficulty, mild difficulty, moderate difficulty, severe difficulty, and unable to do the task. The sum of the responses is divided by the number of responses, and one is subtracted from this value; the final value is multiplied by 25. The formula can be found in the Figure.
Typically, the DASH score is used to monitor upper extremity functionality after musculoskeletal (MSK) surgeries.69 Studies have found the DASH score to be an effective tool in detecting and differentiating both small and large changes in disability over time in patients with MSK disorders.70,71 Although it is only limited to the upper extremity and in those with MSK disorders, we believe the DASH score has considerable potential to be used in wound management, especially for traumatic or complex wounds. Not only will it allow providers to understand how these wounds affect their patients’ lives, but it may also serve as a clinical measurement of how well wounds are healing. Furthermore, comparing DASH scores in patients treated with different matrices will allow providers to compare which products lead to better outcomes.
Conclusions
After reviewing allografts, bioengineered matrices, xenografts, and AFS, we compared indications, advantages, and disadvantages of each ECM. We surmise that the benefits of AFS include diverse utility, antimicrobial activity, low immunogenicity, faster healing times, and cosmetic superiority. It is important to note that AFS can produce a potential cost burden as it is not always covered by insurance and is not plentiful or easily accessible in some parts of the world. However, these problems will likely be solved as current studies continue to produce positive patient data and further investigate other types of fish species and the use of AFS as combination therapy with other ECMs.
Bioengineered matrices and porcine xenografts are other great therapeutic options and are viable alternatives to AFS. Their benefits include antimicrobial properties and low immunogenicity, both of which are desired in an effective skin graft. Furthermore, they can be utilized in a variety of situations, are cost effective, and readily available. One downside is that these products require longer healing times and do not produce the same cosmesis as AFS.
Lastly, allografts, whether they are glycerol or cryopreserved, are also a viable choice of treatment. They are clearly better than exposure healing, and while they may not heal as quickly or produce cosmetic results that are as desirable as those seen with AFS, bioengineered matrices, or porcine and bovine xenografts, they still possess the desirable properties of autologous skin while stimulating vascularization and providing an adequate antimicrobial barrier and wound closure. With these conclusions, we intend for ECM selection to require less subjectivity and become more algorithmic in hopes of improving wound healing and increasing positive patient outcomes. Furthermore, we hope this analysis draws attention to the limited number of head-to-head and comparison trials between different ECMs and ultimately spurs further investigation into which ECM is best suited to a particular wound type.
Acknowledgments
Affiliations: 1Department of Medicine, UCLA-Oliveview Medical Center, Sylmar, California; 2The University of Toledo Health Science Campus, Toledo, Ohio; 3Jobst Vascular Institute, ProMedica Health Network, Toledo, Ohio; 4Department of Surgery, Division of Plastic and Reconstructive Surgery, University of Toledo College of Medicine, Toledo, Ohio; 5Department of Internal Medicine, University of Toledo College of Medicine and Life Sciences, Toledo, Ohio
Correspondence: Samuel Stetkevich, MD; sstetkevich@dhs.lacounty.gov
Disclosures: None of the authors has declared a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
1. Sorushanova A, Delgado LM, Wu Z, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater. 2019 Jan;31(1):e1801651. doi:10.1002/adma.201801651
2. Piez KA. History of extracellular matrix: a personal view. Matrix Biol. 1997 Aug;16(3):85-92. doi:10.1016/s0945-053x(97)90037-8
3. Gore MA, De AS. Deceased donor skin allograft banking: response and utilization. Indian J Plast Surg. 2010;43(Suppl):S114-S120. doi:10.4103/0970-0358.70732
4. Kua EH, Goh CQ, Ting Y, Chua A, Song C. Comparing the use of glycerol preserved and cryopreserved allogenic skin for the treatment of severe burns: differences in clinical outcomes and in vitro tissue viability. Cell Tissue Bank. 2012 Jun;13(2):269-279. doi:10.1007/s10561-011-9254-4
5. Calota DR, Nitescu C, Florescu IP, Lascar I. Surgical management of extensive burns treatment using allografts. J Med Life. 2012 Dec 15;5(4):486-490.
6. Yoon C, Lim K, Lee S, Choi Y, Choi Y, Lee J. Comparison between cryopreserved and glycerol-preserved allografts in a partial-thickness porcine wound model. Cell Tissue Bank. 2016 Mar;17(1):21-31. doi:10.1007/s10561-015-9521-x
7. Leicht P, Muchardt O, Jensen M, Alsbjörn BA, Sørensen B. Allograft vs. exposure in the treatment of scalds--a prospective randomized controlled clinical study. Burns Incl Therm Inj. 1989 Feb;15(1):1-3. doi:10.1016/0305-4179(89)90058-2
8. Wang C, Zhang F, Lineaweaver WC. Clinical applications of allograft skin in burn care. Ann Plast Surg. 2020 Mar;84(3S Suppl 2):S158-S160. doi:10.1097/SAP.0000000000002282
9. Ranaweera A. Bioengineered skin. DermNet. Accessed July 5, 2022. https://dermnetnz.org/topics/bioengineered-skin
10. Purdue GF, Hunt JL, Gillespie RW, et al. Biosynthetic skin substitute versus frozen human cadaver allograft for temporary coverage of excised burn wounds. J Trauma. 1987 Feb;27(2):155-157. doi:10.1097/00005373-198702000-00010
11. Austin RE, Merchant N, Shahrokhi S, Jeschke MG. A comparison of Biobrane™ and cadaveric allograft for temporizing the acute burn wound: cost and procedural time. Burns. 2015 Jun;41(4):749-753. doi:10.1016/j.burns.2014.10.003
12. Waymack P, Duff RG, Sabolinski M. The effect of a tissue engineered bilayered living skin analog, over meshed split-thickness autografts on the healing of excised burn wounds. The Apligraf Burn Study Group. Burns. 2000 Nov;26(7):609-619. doi:10.1016/s0305-4179(00)00017-6
13. Gohari S, Gambla C, Healey M, et al. Evaluation of tissue-engineered skin (human skin substitute) and secondary intention healing in the treatment of full thickness wounds after Mohs micrographic or excisional surgery. Dermatol Surg. 2002 Dec;28(12):1107-1114; discussion 1114. doi:10.1046/j.1524-4725.2002.02130.x
14. Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996 Apr;19(4):350-354. doi:10.2337/diacare.19.4.350
15. Greenwood JE. The evolution of acute burn care - retiring the split skin graft. Ann R Coll Surg Engl. 2017;99(6):432-438. doi:10.1308/rcsann.2017.0110
16. Li H, Lim P, Stanley E, et al. Experience with NovoSorb® Biodegradable Temporising Matrix in reconstruction of complex wounds. ANZ J Surg. 2021;91(9):1744-1750. doi:10.1111/ans.16936
17. NovoSorb® BTM: Synthetic polymer for traumatic wound treatment. United States. https://polynovo.com/product-btm/. Published March 2, 2022. Accessed December 1, 2022.
18. Solanki NS, York B, Gao Y, Baker P, Wong She RB. A consecutive case series of defects reconstructed using NovoSorb® Biodegradable Temporising Matrix: initial experience and early results. J Plast Reconstr Aesthet Surg. 2020;73(10):1845-1853. doi:10.1016/j.bjps.2020.05.067
19. Jones JE, Nelson EA. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev. 2007 Apr 18;(2):CD001737. doi:10.1002/14651858.CD001737.pub3
20. Greaves NS, Iqbal SA, Hodgkinson T, et al. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen. 2015 Jul-Aug;23(4):483-494. doi:10.1111/wrr.12308
21. Vloemans AF, Soesman AM, Suijker M, Kreis RW, Middelkoop E. A randomised clinical trial comparing a hydrocolloid-derived dressing and glycerol preserved allograft skin in the management of partial thickness burns. Burns. 2003 Nov;29(7):702-710. doi:10.1016/s0305-4179(03)00161-x
22. Nicoletti G, Tresoldi MM, Malovini A, Visaggio M, Faga A, Scevola S. Versatile use of dermal substitutes: a retrospective survey of 127 consecutive cases. Indian J Plast Surg. 2018 Jan-Apr;51(1):46-53. doi:10.4103/ijps.IJPS_217_17
23. Caravaggi C, Grigoletto F, Scuderi N. Wound bed preparation with a dermal substitute (Hyalomatrix® PA) facilitates re-epithelialization and healing: results of a multicenter, prospective, observational study on complex chronic ulcers (The FAST Study). Wounds. 2011 Aug;23(8):228-235.
24. Nicoletti G, Brenta F, Bleve M, Pellegatta T, Malovini A, Faga A, Perugini P. Long-term in vivo assessment of bioengineered skin substitutes: a clinical study. J Tissue Eng Regen Med. 2015 Apr;9(4):460-468. doi:10.1002/term.1939
25. Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs. 1997 Nov;21(11):1203-1210. doi:10.1111/j.1525-1594.1997.tb00476.x
26. Dinh TL, Veves A. The efficacy of Apligraf in the treatment of diabetic foot ulcers. Plast Reconstr Surg. 2006 Jun;117(7 Suppl):152S-157S; discussion 158S-159S. doi:10.1097/01.prs.0000222534.79915.d3
27. Buchberger B, Follmann M, Freyer D, Huppertz H, Ehm A, Wasem J. The importance of growth factors for the treatment of chronic wounds in the case of diabetic foot ulcers. GMS Health Technol Assess. 2010 Sep 1;6:Doc12. doi:10.3205/hta000090
28. Marston WA. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices. 2004 Sep;1(1):21-31. doi:10.1586/17434440.1.1.21
29. Buchberger B, Follmann M, Freyer D, Huppertz H, Ehm A, Wasem J. The evidence for the use of growth factors and active skin substitutes for the treatment of non-infected diabetic foot ulcers (DFU): a health technology assessment (HTA). Exp Clin Endocrinol Diabetes. 2011 Sep;119(8):472-479. doi:10.1055/s-0031-1279713
30. Yamamoto T, Iwase H, King TW, Hara H, Cooper DKC. Skin xenotransplantation: historical review and clinical potential. Burns. 2018;44(7):1738-1749. doi:10.1016/j.burns.2018.02.029
31. Sokolic IH, Farpour A, Ulin AW, et al. The use of heterograft skin as a biological dressing. Surg Forum. 1960;10:847-849.
32. Chang WH, Gomez NH, Edelstein LM. Use of lyophilised pig skin for donor site cover. Br J Plast Surg. 1973;26(2):147-149. doi:10.1016/s0007-1226(73)80008-6
33. Still JM, Law EJ, Craft-Coffman B. An evaluation of excision with application of autografts or porcine xenografts within 24 hours of burn injury. Ann Plast Surg. 1996;36(2):176-179. doi:10.1097/00000637-199602000-00013
34. Aronoff M, Fleishman P, Simon DL. Experience in the application of porcine xenografts to split-graft donor sites. J Trauma. 1976;16(4):280-283. doi:10.1097/00005373-197604000-00005
35. Chang WH, Gomez NH, Edelstein LM. Use of lyophilised pig skin for donor site cover. Br J Plast Surg. 1973;26(2):147-149. doi:10.1016/s0007-1226(73)80008-6
36. Green D, Law E, Still JM. An approach to the management of toxic epidermal necrolysis in a burn centre. Burns. 1993;19(5):411-414. doi:10.1016/0305-4179(93)90063-e
37. Chiu T, Burd A. “Xenograft” dressing in the treatment of burns. Clin Dermatol. 2005;23:419-423. doi:10.1016/j.clindermatol.2004.07.027
38. Leto Barone AA, Mastroianni M, Farkash EA, et al. Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns. 2015;41(3):565-574. doi:10.1016/j.burns.2014.09.003
39. Galili U, Wang L, LaTemple DC, Radic MZ. The natural anti-Gal antibody. Subcell Biochem. 1999;32:79-106. doi:10.1007/978-1-4615-4771-6_4
40. Ersek RA, Hachen HJ. Porcine xenografts in the treatment of pressure ulcers. Ann Plast Surg. 1980;5(6):464-470. doi:10.1097/00000637-198012000-00009
41. Rappaport I, Pepino AT, Dietrick W. Early use of xenografts as a biologic dressing in burn trauma. Am J Surg. 1970;120(2):144-148. doi:10.1016/s0002-9610(70)80102-7
42. Snyderman R, Reuven K, Miller D, et al. Prolonged skin homograft and heterograft survival in patients with neoplastic disease. Plast Reconstr Surg. 1960;26(4):373-377.
43. Jarman-Smith ML, Bodamyali T, Stevens C, Howell JA, Horrocks M, Chaudhuri JB. Porcine collagen crosslinking, degradation and its capability for fibroblast adhesion and proliferation. J Mater Sci Mater Med. 2004;15(8):925-932. doi:10.1023/B:JMSM.0000036281.47596.cc
44. Hermans MH. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference?. Burns. 2014;40(3):408-415. doi:10.1016/j.burns.2013.08.020
45. Cazzell SM, Lange DL, Dickerson JE Jr, Slade HB. The management of diabetic foot ulcers with porcine small intestine submucosa tri-layer matrix: a randomized controlled trial. Adv Wound Care (New Rochelle). 2015;4(12):711-718. doi:10.1089/wound.2015.0645
46. Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13(5):377-383. doi:10.1016/s1084952102000940
47. AbouIssa A, Mari W, Simman R. Clinical usage of an extracellular, collagen-rich matrix: a case series. Wounds. 2015;27(11):313-318.
48. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5(1):1-13. doi:10.1016/j.actbio.2008.09.013
49. Shi L, Ronfard V. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): a mini review. Int J Burns Trauma. 2013;3(4):173-179.
50. Lee YC. Early heterografting of partial-thickness burns. J Trauma. 1972;12(9):818-820. doi:10.1097/00005373-197209000-00012
51. Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26(4):507-523. doi:10.1016/j.cpm.2009.08.001
52. Rennert RC, Sorkin M, Garg RK, Januszyk M, Gurtner GC. Cellular response to a novel fetal acellular collagen matrix: implications for tissue regeneration. Int J Biomater. 2013;2013:527957. doi:10.1155/2013/527957
53. Lullove E. Acellular fetal bovine dermal matrix in the treatment of nonhealing wounds in patients with complex comorbidities. J Am Podiatr Med Assoc. 2012;102(3):233-239. doi:10.7547/1020233
54. Brodsky B, Ramshaw JA. The collagen triple-helix structure. Matrix Biol. 1997;15(8-9):545-554. doi:10.1016/s0945-053x(97)90030-5
55. Kavros SJ, Dutra T, Gonzalez-Cruz R, et al. The use of PriMatrix, a fetal bovine acellular dermal matrix, in healing chronic diabetic foot ulcers: a prospective multicenter study. Adv Skin Wound Care. 2014;27(8):356-362. doi:10.1097/01.ASW.0000451891.87020.69
56. Lantis JC, Snyder R, Reyzelman AM, et al. Fetal bovine acellular dermal matrix for the closure of diabetic foot ulcers: a prospective randomised controlled trial. J Wound Care. 2021;30(Sup7):S18-S27. doi:10.12968/jowc.2021.30.Sup7.S18
57. Luze H, Nischwitz SP, Smolle C, Zrim R, Kamolz L-P. The use of acellular fish skin grafts in burn wound management—a systematic review. Medicina. 2022;58:912. doi:10.3390/medicina58070912
58. Dussoyer M, Michopoulou A, Rousselle P. Decellularized scaffolds for skin repair and regeneration. Appl Sci. 2020;10(10):3435. doi:10.3390/app10103435
59. Pan SC. Burn blister fluids in the neovascularization stage of burn wound healing: a comparison between superficial and deep partial-thickness burn wounds. Burns Trauma. 2013;1(1):27-31. doi:10.4103/2321-3868.113332
60. Júnior EML, Filho MODM, Costa BA, et al. Innovative burn treatment using tilapia skin as a xenograft: a phase II randomized controlled trial. J Burn Care Res. 2020;41:585-592. doi:10.1093/jbcr/irz205
61. Wallner C, Holtermann J, Drysch M, et al. The use of intact fish skin as a novel treatment method for deep dermal burns following enzymatic debridement: a retrospective case-control study. Eur Burn J. 2022;3(1):43-55. doi:10.3390/ebj3010006
62. Michael S, Winters C, Khan M. Acellular fish skin graft use for diabetic lower extremity wound healing: a retrospective study of 58 ulcerations and a literature review. Wounds. 2019 Oct;31(10):262-268.
63. Yang CK, Polanco TO, Lantis JC 2nd. A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds. 2016 Apr;28(4):112-118.
64. Coraça-Huber DC, Steixner S, Wurm A, Nogler M. Antibacterial and anti-biofilm activity of omega-3 polyunsaturated fatty acids against periprosthetic joint infections-isolated multi-drug resistant strains. Biomedicines. 2021;9(4):334. doi:10.3390/biomedicines9040334
65. Kim T-H, Park J-H, Jeong H-G, Wee S-Y. The utility of novel fish-skin derived acellular dermal matrix (Kerecis) as a wound dressing material. J Wound Manag Res. 2021;17(1): 39-47. doi:10.22467/jwmr.2020.01228
66. Magnússon S, Baldursson BT, Kjartansson H, et al. Affrumað roð: eðliseiginleikar sem styðja vefjaviðgerð [Decellularized fish skin: characteristics that support tissue repair]. Laeknabladid. 2015;101(12):567-573. doi:10.17992/lbl.2015.12.54
67. Ahuja RB, Goswami P. Cost of providing inpatient burn care in a tertiary, teaching, hospital of North India. Burns 2013;39:558-564. doi:10.1016/j.burns.2013.01.013
68. Seth N, Chopra D, Lev-Tov H. Fish skin grafts with omega-3 for treatment of chronic wounds: exploring the role of omega-3 fatty acids in wound healing and a review of clinical healing outcomes. Surg Technol Int. 2022 May 19;40:38-46. doi:10.52198/22.STI.40.WH1494
69. Ido Y, Uchiyama S, Nakamura K, et al. Postoperative improvement in DASH score, clinical findings, and nerve conduction velocity in patients with cubital tunnel syndrome. Sci Rep. 2016;6:27497. doi:10.1038/srep27497
70. Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003:4:11. doi:10.1186/1471-2474-4-11
71. Ogura K, Yakoub MA, Christ AB, et al. The critical difference in the DASH (Disabilities of the Arm, Shoulder, and Hand) outcome measure after essential upper extremity tumor surgery. J Shoulder Elbow Surg. 2021;30(9):e602-e609. doi:10.1016/j.jse.2020.11.027