Piscine-Derived Acellular Dermal Matrix in Upper Extremity Reconstruction
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. Wound reconstruction involving exposed critical structures, especially in medically complex patients and in those who are at high risk of loss to follow-up, presents a unique challenge to surgeons. The use of acellular dermal matrix (ADM) has augmented our ability to address these wounds safely and with minimal morbidity. A piscine ADM, the Kerecis Omega3 Wound, has shown promise in the treatment of chronic wounds and burns. In this study, we describe the use of the Omega3 Wound in reconstruction of upper extremity wounds, including those with exposed critical structures.
Methods. From 2019 to 2021, 11 consecutive patients with upper extremity wounds, including 9 with exposed critical structures, were queried. Clinical data was tabulated to evaluate outcomes.
Results. Etiology of the wounds included burns, trauma, infection, and oncologic resection. Wound surface area was 150 ± 42 cm2. Time to incorporation of the Omega3 Wound was 2 weeks, and the vital structures of previously critical wounds were covered with granulation tissue. Clinically and histologically, the ADM became granulation tissue once incorporated. The average time from application to discharge was 6 days, with 3 patients discharged on the day of application. Skin grafting was subsequently performed at an outpatient surgical center, with 3 weeks as the average time to skin grafting.
Conclusions. The Omega3 Wound allows for complete biologic integration and vascularized wound coverage that accepts a skin graft. Patients can be discharged after application and managed at outpatient facilities. This ADM is safe and well tolerated by patients (including those who are diabetic and immunocompromised) for coverage of upper extremity wounds with exposed critical structures.
Introduction
The management of upper extremity wounds presents a unique challenge because of the high concentration of critical structures that lie just below the surface of the skin. Although full-thickness coverage is standard care for wounds of this type, the decision of how to intervene must be weighed against the patient’s overall medical status and comorbidities. Patients with risk factors for reconstructive failure, such as malnutrition, diabetes, and immunocompromise, pose a particular challenge. Another important subset of patients to consider are those who are at high risk of loss to follow-up. Among lower extremity patients, risk factors for loss to follow-up include male gender, lower income status, and living farther than 20 minutes from the hospital.1 New Mexico ranks 49th in median household income, and as the only level 1 trauma center in the fifth largest state, our patient population faces many challenges to adequate follow-up.2
The use of acellular dermal matrices (ADM) can be an effective off-the-shelf option for high-risk patients. By converting a critical wound to one that heals secondarily or supports a skin graft, ADMs obviate the need for complex reconstructive procedures. This strategy reduces costs, minimizes or eliminates operative time, and allows for patients to be managed as outpatients, the last of which is a key consideration, and no more pressing than in the COVID-19 pandemic.3
The Omega3 Wound (Kerecis) is an ADM derived from Icelandic cod. It is minimally processed, allowing for the removal of all piscine cells while retaining many extracellular matrix structures and molecules including Omega-3 fatty acids, which are thought to be antimicrobial and help facilitate wound healing. Unlike mammalian tissue, a gentler processing is possible because there are no known diseases that pass between North Atlantic cod and humans.3,4 This type of processing decreases the likelihood of unintentional ADM crosslinking, which allows for improved fibroblast migration through its 3-dimensional (3D) structure. This piscine-derived ADM has previously been described in cases of lower extremity and foot wounds associated with diabetes and vascular insufficiency, and in cutaneous burns.5-9 It has also been described as helping resurface mucosal surfaces, specifically in mucogingival procedures.10 Additionally, there are 2 case reports describing the use of this ADM in the upper extremity.11,12 To our knowledge, this is the first series detailing its use in the critical defects of the upper extremity.
Our objective was to determine the efficacy of the Omega3 Wound in upper extremity wounds with soft tissue loss, including those with exposed critical structures. We hypothesized that this ADM would promote healing in upper extremity wounds with minimal risk to the patient while facilitating the maturation of a critical wound into one suitable for skin grafting or secondary epithelialization.
Methods and Materials
This was a retrospective case series performed with Institutional Review Board approval at the University of New Mexico Hospital. Inclusion criteria were patients with upper extremity wounds that were not suitable for skin grafting who underwent ADM placement from January 1, 2019, to December 31, 2021. Our exclusion criteria were patients younger than 18 years and patients with a white fish allergy, as the ADM is derived from Icelandic cod. Eleven consecutive patients were identified within the study period.
In our protocol, the ADM was prepared, applied, and bolstered with negative pressure wound therapy following debridement of the wounds. Wounds were measured in square centimeters. The mean and standard error of the mean were calculated for all cases.
Following application, stable patients were discharged either home or to other facilities, depending on their medical needs. The patients had their operative site checked weekly to evaluate the wound bed. The upper extremities either underwent skin grafting or were allowed to heal secondarily.
Hospitalization time was defined as the time from ADM application to patient discharge from the inpatient facility. Time to incorporation was defined as the time from ADM application to 90% ADM conversion to granulation tissue in the wound bed at the time of the weekly dressing change. Time to skin grafting was defined as the time from application of the ADM to application of a skin graft. All values had their mean calculated with a standard error of the mean.
One patient had a punch biopsy performed prior to skin grafting, allowing for histological evaluation of the wound once the ADM was incorporated into the wound bed, which was obtained 16 days after the Omega3 Wound application.
Results
Patient demographics and wound etiologies are summarized in Table 1. The wound etiologies were burns, trauma, infection, and oncologic resection. There were exposed critical structures (including tendons and nerves) in 9 of the 11 patients. All patients had comorbidities, including 5 patients with polysubstance use disorder, 4 patients with diabetes, 2 patients with renal transplants, and 2 patients with autoimmune disease. The patients’ ages ranged from 19 to 67 years.
Table 1: Patient Demographics and Etiologies of Wounds

The outcomes are summarized in Table 2. The wound surface areas ranged from 12 to 350 cm2. The mean size was 150 ± 42 cm2. The time to incorporation of the Omega3 Wound was 2 ± 0 weeks. At the time of incorporation, previously critical wounds had their vital structures covered with granulation tissue. Skin grafting was performed at an outpatient surgical center, with a mean time to skin grafting of 3 ± 0 weeks. Six representative cases of this process are included (Figures 1-6).
Table 2. Outcomes



Figure 1. Case 3: (A) A 36-year-old man with a history of tobacco and polysubstance abuse had a traumatic wound of the dorsal hand and exposed extensor tendons. (B) The wound shown 3 weeks after ADM placement, at the time of split-thickness skin grafting. (C) The skin graft shown at the time of dressing takedown, 2 weeks after grafting.

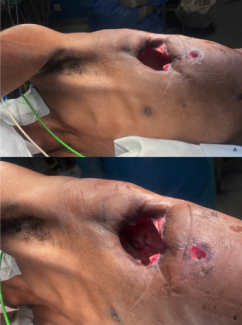
Figure 2. Case 4: (A) A 55-year-old woman had a necrotizing soft tissue infection with exposed tendons in the forearm and dorsum of the hand. (B) The wound shown 2 weeks after placement of the ADM, at the time of skin grafting. (C) The wound shown 3 weeks after skin grafting.

Figure 3. Case 10: A 66-year-old woman with a history of renal transplantation, on immunosuppressive medication, and with systemic lupus erythematosus, underwent resection of a squamous cell carcinoma of the dorsal/radial wrist. (A) She needed subsequent re-resection because of positive margins, which resulted in exposed extensor tendons at the wrist, and which were covered with the ADM. The patient was lost to follow-up but returned for the next stage of her reconstruction 5 weeks later. (B) The wound bed was healthy and there were no longer any exposed structures. (C) The patient had a full thickness skin graft, which healed nicely, and is shown 2 months post-grafting.

Figure 4. Case 11: (A) A 67-year-old man with polysubstance abuse suffered a large traumatic degloving of the dorsal forearm and exposed extensor tendons. The patient had ADM placement, and 2 weeks postoperatively was found to have an infection. (B) There was no loss of the ADM, and the infection resolved after 2 washouts. At the time of second washout, the ADM was placed again over the remaining exposed tendon. The patient was skin grafted approximately 4 weeks after the initial procedure, (C) with the skin graft healing well 2 weeks afterwards.
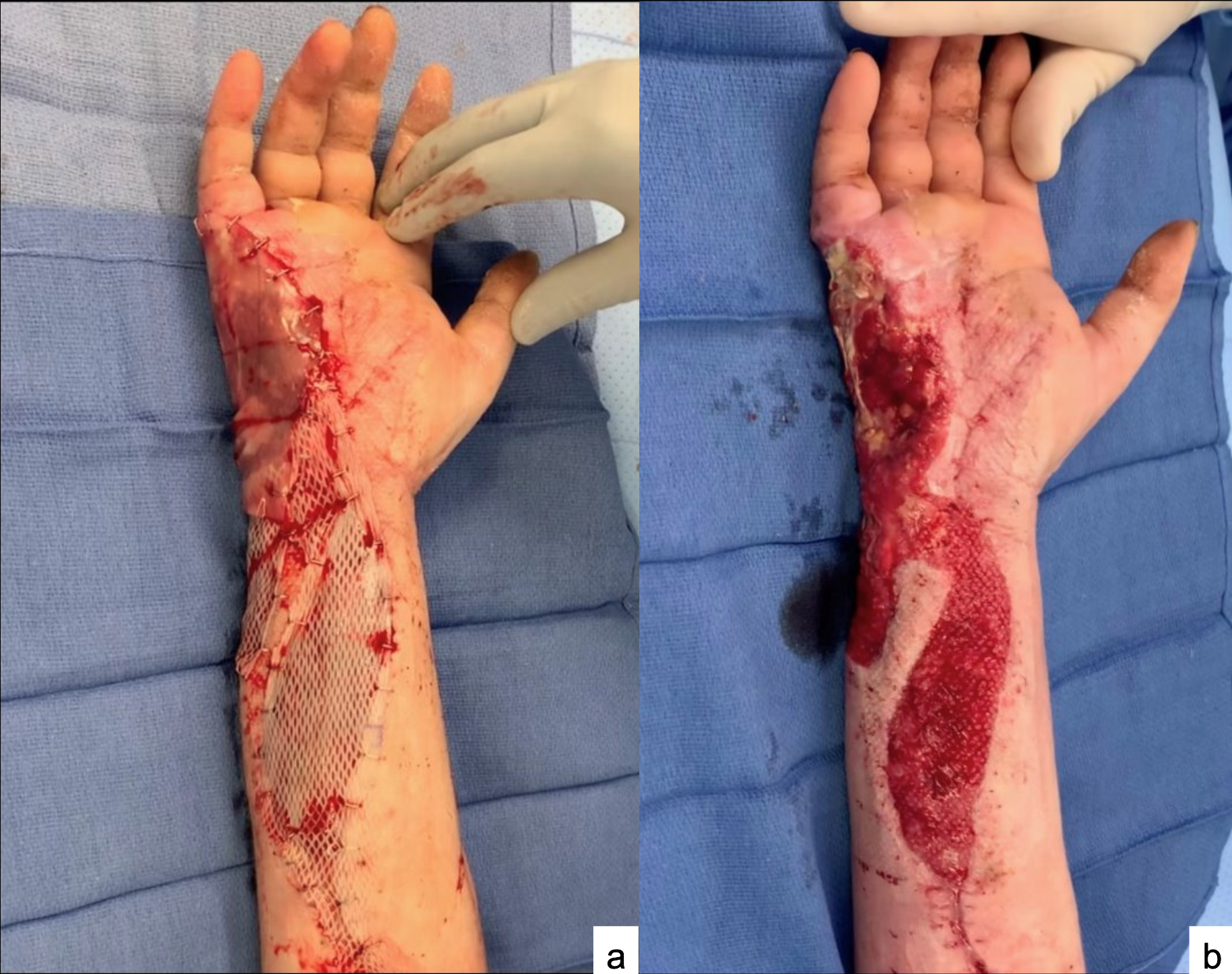
Figure 5. Case 2: A 41-year-old man with a history of polysubstance abuse with necrotizing fasciitis, status post debridement. (A) The patient had a bilaminate neodermis and the Omega3 Wound placed. (B) Two weeks after placement of these products, the patient had loss of the neodermis, however he had full incorporation of the adjacent Omega3 Wound.

Figure 6. Case 6: A 31-year-old woman with history of polysubstance abuse was involved in a polytrauma that resulted in a traumatic degloving injury of the palm. (A) She underwent a debridement and placement of the ADM. (B) She underwent split-thickness skin grafting once the ADM was incorporated at 2 weeks. Just prior to skin grafting, she had a punch biopsy of the wound bed performed, which was sent for histopathologic evaluation. (C) The skin graft started to incorporate at the first dressing change, however, she was lost to follow-up and further photos were unavailable. When contacted over the telephone, the patient stated she had no issues and is fully healed, and she had the surgical staples removed by a local physician.
In Case 3 (Figure 1), the patient developed an abscess and flexor tenosynovitis on the volar palm, which was recognized at the time of the proposed skin grafting. Despite the infection, the patient had no loss of the Omega3 Wound, which was placed on the dorsum of his hand. It was noted to have fully incorporated, covering exposed extensor tendon (Figure 1B). He underwent incision and drainage, and 1 week later underwent skin grafting.
Case 11 (Figure 4) was found to have an infection under the negative pressure wound dressing at the time of the proposed skin grafting. As a result, the patient underwent 2 irrigation and debridement procedures. Despite the infection and washouts, the ADM continued to promote development and retention of vascularized soft tissue over the exposed tendon in the wound bed. Once the wound bed was no longer contaminated, the patient underwent a second application of the ADM, which incorporated and covered the remaining exposed tendon and provided a healthy wound bed for skin grafting 4 weeks postoperatively.
One patient (Case 2) had a bilaminate neodermis placed adjacent to the ADM. At the second dressing change, it was found that the neodermis was infected and completely lost; however, the ADM had still incorporated adjacent to the infected tissue and remained healthy (Figure 5). This patient was lost to follow-up after transfer from our inpatient facility.
One patient (Case 7) with fourth-degree burns had incorporation of the ADM at 2 weeks; however, his hand was nonfunctional due to the severity and extent of the burns. He elected to undergo transradial amputation with targeted muscle reinnervation with the goal of receiving a myoelectric prosthesis in the future.
A punch biopsy was obtained at the time of full incorporation for Case 6 (Figure 6). This biopsy demonstrated a giant cell reaction surrounding the foreign body (the ADM) with dermal acute inflammation with granulation tissue (Figure 7). The angulated vasculature with interposed dermal inflammation was consistent with the robust granulation the patient demonstrated clinically.

Figure 7. Histologic slide from Case 6: (A) Enhanced magnification of a punch biopsy obtained from Case 6 at the time of full incorporation for Case 6, demonstrating dermal acute inflammation with granulation tissue and multinuclear giant cell reacting to the Omega3 Wound. (B) Full view of punch biopsy: wound surface is superior, wound base is inferior.
Discussion
Extensive zones of injury make local reconstruction of traumatic defects challenging. Given the paucity of soft tissue reservoirs in the distal extremities, more distant regional flaps or free flap anastomosis outside the zone of injury are often required for successful reconstruction. In highly morbid, poorly optimized patients, this strategy can be imprudent. In our hands, the Omega3 Wound routinely circumvents the need for complex soft tissue reconstruction for a multitude of wound types, reduces inpatient hospitalization and demand on operative time, and frees patients from burdensome postoperative protocols.
The Omega3 Wound is a piscine-derived ADM with clinical success, as described in cases of lower extremity wounds. A previous case series examined the ADM in the setting of diabetic foot ulcers, where the authors demonstrated that the product can help facilitate healing in a chronic wound with multiple applications of the ADM.5,6,8 In a trial of chronic diabetic wounds, ADM application was superior to offloading and local wound care; specifically, 67% of the ADM patients achieved complete wound closure at 12 weeks, compared with only 32% of the local wound care arm. In the same study, at 6 weeks, there was a 73% wound surface area reduction with the use of the ADM vs 41% for patients treated with local wound care. Studies have also shown that ADM application can help facilitate more rapid healing in partial-thickness burns.9 When compared with fetal bovine dermis, Omega3 Wound application resulted in greater re-epithelialization (50% with Omega3 Wound vs 23.5% with bovine dermis) and had significantly smaller wound size (93.1% with Omega3 Wound vs 106.7% with bovine dermis) at 14 days post-application. Besides burns and chronic lower extremity wounds, there are very few settings where Omega3 Wound has been used, including a single study on mucogingival procedures and one case report describing use in the upper extremity with exposed critical structures.10,11
In our patient population, 9 of the 11 patients had exposed critical structures (including tendons and nerves), and the ADM incorporated over these structures. Seven of these patients had their wounds completely closed: 6 with skin grafting, and 1 closed secondarily. In our clinical experience, the ADM incorporates quickly (2-3 weeks) and produces a vascularized wound bed that is amenable to skin grafting (also demonstrated histologically in Figure 7). Notwithstanding the high proportion of patients with risk factors for poor healing outcomes and loss at follow-up, we found the Omega3 Wound to be efficacious. For patients who are lost to follow-up, their wounds (which are no longer critical) can be managed by local wound care alone, such as in Case 1.
Many of the cases in this study involved traumatic and contaminated wounds. Despite the development of adjacent infection in 3 traumatic cases, the Omega3 Wound continued to incorporate and promote granulation tissue formation (Figures 1, 4, and 5). In case 3, the palmar aspect of the hand developed an infection; however, this did not affect the dorsal wound. In case 2, the bilaminate neodermis that was applied got infected; however, the ADM was still able to incorporate despite adjacent infection (Figure 5B). Similarly, in case 11, the incorporated ADM was not lost, despite the wound bed infection. There is data that the Omega3 Wound is more resistant to infection than chorion and amnion, which may be a potential advantage over other off-the-shelf products.13 In 1 study, the ADM had superior 3D ingrowth, which was attributed to a more porous microstructure, and a better bacterial barrier than dehydrated human amnion/chorion membrane at 24 to 48 hours. The antimicrobial property may be partially due to the Omega3 fatty acids that are retained in the ADM. These may explain why we found the Omega3 Wound to incorporate quickly and resist adjacent infection; plus, it has anti-inflammatory properties that can be beneficial in a hostile wound environment.14
In terms of technical considerations, our protocol has changed with clinical experience. Initially, we used a bolster dressing with non-adherent gauze and surgical lubricant to keep the wound bed moist. When we switched to negative pressure wound therapy, it functionally served to increase the migration of fibroblasts into the Omega3 Wound, and we found the granulation tissue to be more robust clinically. Patients also tolerated negative pressure wound therapy better with fewer dressing changes. When preparing the wound bed, healthy, bleeding tissue is necessary for more rapid incorporation. Unlike other products that only have single-layer contact with the bed, the Omega3 Wound can be stacked 2 or 3 times in deeper wounds, making the newly formed granulation tissue flush with the skin surface. We have also had success with applying the ADM at bedside with oral sedation or local anesthetic, thereby saving operative time.
Limitations
This study is a retrospective series of cases, so it is limited by the nature of the study. There are no controls to compare to, and many patients were lost to follow-up. Many of our patients are low income and must travel from long distances (>100 miles) for care. There is also limited discussion in the published literature about this type of reconstruction. Future prospective studies comparing this product to other ADMs, neodermis, and reconstructive procedures would be beneficial. We hope to see studies with more patients and with longer-term follow-up in order to compare our experience with that of others.
Conclusions
The Kerecis Omega3 Wound ADM is safe, well tolerated, and allows for complete biologic integration and vascularized wound coverage that will accept a skin graft. It converts critical wounds to ones amenable to either skin grafting or local wound care, and appears to be resistant to infection. Finally, the Omega3 Wound is a useful tool for reconstruction in patients who are poor surgical candidates, either for medical reasons or because of socioeconomic factors that may limit their ability to access appropriate postoperative care. By treating complex problems with simple solutions, we have the potential to reduce patient morbidity and the burden on the hospital system as a whole.
Acknowledgments
Authors: Shawhin Shahriari, MD, MA, MS1,2; Cees Whisonant, MD3; Joseph Kuhn, MD1; Tyler Chavez, MD4; Joshua Harrison, MD1; Casey McDonald, MD5; Adam Schwartz, MD1; Jolee Suddock, MD6; Elizabeth Mikola, MD4; Gregory Borah, MD, DMD1
Affiliations: 1University of New Mexico, Department of Surgery, Division of Plastic, Reconstructive, Hand and Burn Surgery, Albuquerque, New Mexico; 2INTEGRIS Health, Department of Orthopedic Surgery, Oklahoma City, Oklahoma; 3Creighton University – Phoenix, Department of Surgery, Phoenix, Arizona; 4University of New Mexico, Department of Orthopaedic Surgery, Albuquerque, New Mexico; 5University of Texas Health – San Antonio, Department of Orthopaedics, San Antonio Texas; 6University of New Mexico, Department of Pathology, Albuquerque, New Mexico
Correspondence: Shawhin Shahriari MD, MS, MS; Shawhin7@gmail.com
Ethics: IRB # 22-040
Funding: No sources of support were used for this study.
Disclosures: The authors disclose no relevant financial or nonfinancial interests.
References
1. Sharif-Askary B, Zolper EG, Deldar R, et al. Risk factors for loss to follow-up in the lower extremity limb salvage population. Plast Reconstr Surg. 2021;148(4):883-893. doi:10.1097/PRS.0000000000008356
2. Quick Facts, New Mexico. United States Census Bureau. Accessed June 23, 2022. https://www.census.gov/quickfacts/fact/table/NM/INC110219
3. Da L-C, Huang Y-Z, Xie H-Q, Zheng B-H, Huang Y-C, Du S-R. Membranous extracellular matrix-based scaffolds for skin wound healing. Pharmaceutics. 2021;13(11):1796. doi:10.3390/pharmaceutics13111796
4. Holl J, Kowalewski C, Zimek Z, et al. Chronic diabetic wounds and their treatment with skin substitutes. Cells. 2021;10(3):655. doi:10.3390/cells10030655
5. Woodrow T, Chant T, Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: a prospective evaluation. J Wound Care. 2019;28(2):76-80. doi:10.12968/jowc.2019.28.2.76
6. Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis Ii JC. A multicenter, blinded, randomized controlled clinical trial evaluating the effect of omega-3-rich fish skin in the treatment of chronic, nonresponsive diabetic foot ulcers. Wounds. 2021;33(7):169-177. doi:10.25270/wnds/2021.1691777. Baldursson BT, Kjartansson H, Konrádsdóttir F, Gudnason P, Sigurjonsson GF, Lund SH. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Low Extrem Wounds. 2015;14(1):37-43. doi:10.1177/1534734615573661
8. Michael S, Winters C, Khan M. Acellular fish skin graft use for diabetic lower extremity wound healing: a retrospective study of 58 ulcerations and a literature review. Wounds. 2019;31(10):262-268.
9. Stone R II, Saathoff EC, Larson DA, et al. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int J Mol Sci. 2021;22(4):1590. doi:10.3390/ijms22041590
10. Dragan IF, Garcia H AA, Malik R, Karimbux NY. One-year outcomes of a piscine soft tissue alternative used in mucogingival procedures: a clinical case series. Int J Periodontics Restorative Dent. 2020;40(4):603-609. doi:10.11607/prd.4165
11. Shahriari SR, Ederle AC, Whisonant C, Harrison J, Borah G, Shetty A. Successful upper extremity limb salvage using cellular- and tissue-based products in a patient with uncontrolled diabetes. Wounds. 2022;34(10):E104-E107. doi:10.25270/wnds/21071
12. Shahriari SRK, Harrison JL, Clarke TN, et al. Acellular piscine dermis for pediatric hand burn reconstruction. Plast Reconstr Surg Glob Open. 2024;12(7):e5889. doi:10.1097/GOX.0000000000005889
13. Magnusson S, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson GF. Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: implications for tissue preservation in combat casualty care. Mil Med. 2017;182(S1):383-388. doi:10.7205/MILMED-D-16-00142
14. Shahriari S, Ensign E, Huang S, Harrison J, Whisonant C, Aubin-Lemay C. Successful treatment of wounds from nonuremic calciphylaxis with acellular piscine dermis. Plast Reconstr Surg Glob Open. 2023;11(7):e5120. doi: 10.1097/GOX.0000000000005120