Incidental Breast Carcinoma in Reduction Mammoplasty: A Systematic Review
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. Breast reduction is one of the most common plastic surgeries, with more than 40000 procedures performed in the United States annually. As breast reductions remove a portion of the breast and distort the anatomy, plastic surgeons need to be mindful of the possibility of breast cancer. In this study, we sought to review the available literature on breast cancer workup for patients undergoing reduction mammoplasties.
Methods. We queried the PubMed (National Institutes of Health) and Embase (Elsevier) databases to identify studies discussing breast cancer workup before breast reduction via preoperative imaging and/or at the time of surgery via histopathologic evaluation of breast specimens. Two individual reviewers screened the titles and abstracts for relevance. We extracted data on the outcomes of preoperative imaging and histopathologic evaluation of breast reduction specimens.
Results. Twenty-three articles published between 1996 and 2022 met the inclusion/exclusion criteria. Two studies evaluated only the role of preoperative imaging and reported a biopsy rate of 3.7% to 5.1% based on imaging findings. Three studies discussed only the role of histopathologic evaluation without mentioning the preoperative imaging requirements from the patients. For the remaining 18 studies, the rate of incidental breast cancer from breast reduction specimens was 0.0% to 2.0%. All studies recommended universal histopathologic evaluation of breast specimens.
Conclusions. In this review, we found unanimous recommendations for performing histopathologic evaluation of breast reduction samples, consistent with the 2022 American Society of Plastic Surgeons clinical practice guideline. Further research is still required to determine the optimal preoperative imaging approach for breast reduction.
Introduction
Macromastia can negatively affect the health of women. Physically, macromastia causes neck, shoulder, and back pain, as well as dermatitis in the skin folds.1,2 Functionally, macromastia can impair women’s confidence and quality of life.2,3 As a solution, breast reduction is one of the most common plastic surgery procedures. Annually, more than 40000 breast reductions are performed in the United States, according to the American Society of Plastic Surgeons (ASPS).4 Because a portion of the breast is removed in breast reduction, plastic surgeons need to be mindful of the possibility of breast cancer.
Despite the high volume of breast reductions performed each year, breast cancer workup varies widely across plastic surgery practices. In general, the workup involves preoperative imaging and/or histopathologic evaluation of breast reduction specimens. Preoperative imaging before breast reduction aims to detect any abnormal radiological findings before the surgery and triage potential breast cancers for appropriate workups.5 National guidelines recommend breast cancer screening with mammography starting at 40 to 50 years of age for women with average risk.6-8 A Continuing Medical Education article by the ASPS recommended obtaining preoperative mammography considering specific patient risk factors, such as age, past medical history, and family history.9 Preoperative imaging also lowers the odds of incidental breast cancer at the time of breast reduction.9
Histopathologic evaluation of breast reduction specimens aims to find occult pathologies that were not suggested in the preoperative imaging or clinical breast exams. Due to the benefits of detecting premalignant or malignant breast lesions and reducing patient anxiety, the 2022 ASPS practice guideline recommends that breast tissue from post-menarche women be sent to pathology.10 However, there is a wide range of incidental breast cancer rates reported at the time of breast reduction. Thus, in this study, we aimed to summarize the available literature on the incidence of occult breast cancer identified in patients undergoing reduction mammoplasties.
Methods and Materials
We queried the PubMed (National Institutes of Health) and Embase (Elsevier) databases on February 1, 2023, to identify research articles on breast cancer workup for patients undergoing breast reductions. Our search filter included any articles with titles or abstracts containing a term related to the surgery (breast reduction OR reduction mammoplasty) and a term related to the breast cancer workup (mammogram OR mammography OR breast cancer screening OR biopsy OR pathology OR path). Two independent reviewers, J.H.G. and R.K.A., screened the article titles and abstracts for relevance. The authors adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
From the initial query, our inclusion and exclusion criteria for titles and abstracts were as follows: (1) All full-length articles written in English evaluating breast cancer before or at the time of the procedure were included; (2) Only primary studies (eg, cohort studies, case series, and randomized controlled studies) were included; (3) Articles written in non-English language, conference abstracts, review articles, correspondences, articles with a primary focus on patients with breast cancer, duplicate articles, and research on unrelated topics were excluded; (4) The articles included after screening the titles and abstracts were screened at full length; (5) For multiple articles with the same cohort identified by the author list and study site, only the latest article was included in this study; (6) The references of the full-length articles were also screened to identify potentially relevant article.
From the included full-length articles, we extracted the manuscript title, author, publication year, journal, details in preoperative breast cancer screening, sample size, average (or median) age, imaging and/or histopathologic findings, and study recommendations. If the study included both patients with a history of breast cancer and those without breast cancer, we extracted data specific to the patients without breast cancer history, if possible. If not, we specified the percentage of patients with breast cancer history in each article.
Results
The primary search included 328 articles (144 PubMed and 184 Embase articles, Figure). From the primary search, the titles and abstracts of 129 articles were screened for relevance. Among the 25
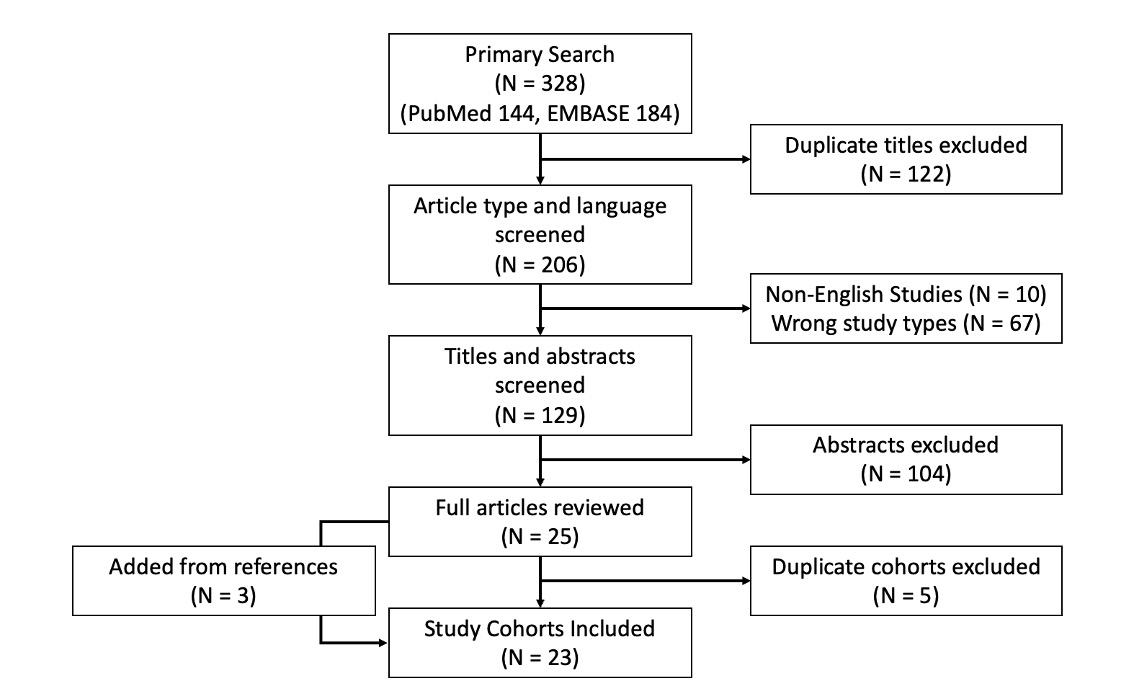
Figure. Study selection flowchart.
Among the included full-length articles, 2 (8.7%) studies discussed only preoperative imaging to screen for breast cancer prior to breast reduction (Table 1). The breast lesion biopsy rate after preoperative imaging was 3.7% to 5.1% (median, 4.4%), and the incidence of breast cancer confirmed with biopsy was 0.0% to 0.2% (median, 0.1%).5,11 Derebaşınlıoğlu et al emphasized the importance of preoperative imaging for women 40 to 59 years old, as proliferative breast lesions were more frequently found in this age group.5 The authors also briefly mentioned that all breast reduction specimens at their institution are sent for histopathologic evaluation.5

HP, histopathologic evaluation; MMG, mammography; US, ultrasound.
Three (13.0%) studies discussed only the histopathologic evaluation of breast reduction specimens without requiring preoperative imaging in the inclusion criteria (Table 2). The rate of detecting occult malignancy was 0.0% to 1.0% (median, 0.5%).12-14 All 3 studies recommended routine histopathologic evaluation of breast reduction specimens. Based on the study results, Hassan et al suggested that routine preoperative imaging for women 35 to 40 years

HP, histopathologic evaluation; MMG, mammography. US, ultrasound.



ACS, American Cancer Society; ASBS, American Society of Breast Surgeons; CBE, clinical breast exam; HP, histopathologic evaluation; MMG, mammography. US, ultrasound.
Discussion
In this review of studies discussing breast cancer surveillance for patients undergoing breast reductions, we report 3 key findings. First, the preoperative biopsy rate based on imaging was 3.7% to 5.1% (median, 4.4%). However, malignancy was found only in 0.0% to 0.2% (median, 0.1%) of the patients. Second, most studies report low rates of incidental breast cancer at the time of breast reduction surgery, with a highest reported rate of 2.0%. Finally, despite the low rates of incidental breast cancer, the included studies unanimously recommend that plastic surgeons send breast reduction specimens for histopathologic evaluation.
National guidelines vary in the recommended age to start breast cancer screening and range from at age 40 to 50 years, and all recommend using mammography for women with average risk.6-8 While there is strong evidence that the use of mammography can reduce breast cancer mortality,33-35 false-positive imaging findings are quite common and have been reported to cause short-term anxiety for patients.36 Based on a cohort of nearly 170000 patients who underwent breast cancer screening, Hubbard et al reported false-positive biopsy recommendation rates of 7.0% for women screened annually and 4.8% for those screened biennially.37 In our review, the 2 studies on preoperative imaging reported similar or lower biopsy rates.5,11 The lower biopsy rate of 3.7%, reported by Derebaşınlıoğlu et al may be due to having younger patients (mean age of 42 years) in the study cohort,5 while Hubbard et al analyzed women with a minimum age of 40 years.37 Most studies in our review included various forms of preoperative imaging requirements, most commonly mammography for patients above an age cutoff and an ultrasound for those below the age cutoff. Some studies mentioned that the authors’ institutional policies require all patients undergoing breast reductions to have preoperative imaging. It is unclear whether these decisions on preoperative imaging are based on evidence or are defensive in nature. Regardless, women undergoing breast reductions should be compliant with breast cancer screening recommendations,16,21 as the surgery could distort the breast anatomy postoperatively.5
The 2022 ASPS clinical practice guideline on reduction mammaplasty recommends that all breast reduction specimens be sent to pathology;10 this was a new addition to the 2012 ASPS clinical practice guideline.38 The 2022 work group assigned “moderate” strength for both evidence quality and recommendation.10 Expected benefits of universal histopathologic evaluation of breast specimens include early detection of breast lesions and reducing patient anxiety.10 However, the authors also noted that benign pathological findings may have a higher associated cost and cause more patient anxiety.10 On a similar note, Srivastava et al reported a higher prevalence of anxiety and depression among women with benign breast diseases compared with their healthy counterparts.39 The practice guideline work group recognized the importance of histopathologic evaluation for breast reductions on patients 40 years or older but did not find evidence suggesting against evaluation for those younger than 40 years
Limitations
Our study has several limitations. First, there were only 2 studies that primarily focused on preoperative imaging as the breast cancer screening modality prior to breast reductions. For the studies that designated specific preoperative imaging guidelines, evidence for the corresponding guidelines was often not cited. Also, several studies were conducted outside of the United States, resulting in various age cutoffs for using mammography vs ultrasound as the primary preoperative imaging modality. For the studies that recommended age-based imaging modality, ultrasound was recommended for younger patients The age cutoffs for using ultrasound to screen for breast cancer were most commonly 30, 35, or 40 years. Some studies recommended using both if either the mammography or ultrasound findings are equivocal. More studies evaluating the cost-effectiveness of different preoperative imaging requirements, especially for women younger than the age set by the national guidelines, are imperative. Perhaps the next iteration of the ASPS clinical practice guidelines could discuss a reasonable approach to screening for breast cancer before breast reduction. Lastly, a few studies included patients with a history of breast cancer on the contralateral side who were undergoing breast reductions. While we were able to extract data specific to patients without breast cancer in these studies, such data extraction was not entirely possible every time.
Conclusions
In this review of 23 studies on breast cancer workup for patients undergoing reduction mammoplasties, we found unanimous recommendations for performing histopathologic evaluation of breast reduction samples, which was consistent with a recommendation by the 2022 ASPS clinical practice guideline. Further research is still required to determine the optimal preoperative imaging approach for breast reduction, especially for women younger than 40 to 50 years.
Acknowledgments
Authors: Jung Ho Gong, MD; Ronald K. Akiki, MD; Rachel Sullivan, MD
Affiliation: Division of Plastic and Reconstructive Surgery, The Warren Alpert Medical School of Brown University, Providence, Rhode Island
Correspondence: Rachel Sullivan, MD; rachelsullivanmd@gmail.com
Disclosures: The authors disclose no financial or other conflicts of interest.
References
1. Gonzalez F, Walton RL, Shafer B, Matory WE Jr, Borah GL. Reduction mammaplasty improves symptoms of macromastia. Plast Reconstr Surg. 1993;91(7):1270-1276. doi:10.1097/00006534-199306000-00013
2. Sigurdson L, Mykhalovskiy E, Kirkland SA, Pallen A. Symptoms and related severity experienced by women with breast hypertrophy. Plast Reconstr Surg. 2007;119(2):481-486. doi:10.1097/01.prs.0000246407.87267.46
3. Guthrie E, Bradbury E, Davenport P, Souza Faria F. Psychosocial status of women requesting breast reduction surgery as compared with a control group of large-breasted women. J Psychosom Res. 1998;45(4):331-339. doi:10.1016/s0022-3999(98)00002-6
4. Breast reduction in young women improves quality of life decades later. American Society of Plastic Surgeons. October 28, 2019. Accessed October 21, 2023. https://www.plasticsurgery.org/news/press-releases/breast-reduction-in-young-women-improves-quality-of-life-decades-later
5. Derebaşınlıoğlu H, Karaca SN. The importance of preoperative imaging methods in reduction mammoplasty. J Plast Reconstr Aesthet Surg. 2022;75(4):1424-1430. doi:10.1016/j.bjps.2021.11.073
6. Breast Cancer: Screening. U. S. Preventative Services Task Force. Published online April 30, 2024. Accessed November 5, 2024. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
7. American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. Updated December 19, 2023. Accessed October 21, 2023. https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
8. Committee on Practice Bulletins—Gynecology. Breast cancer risk assessment and screening in average-risk women. American College of Obstetricians and Gynecologists. July 2017. Accessed October 21, 2023. https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2017/07/breast-cancer-risk-assessment-and-screening-in-average-risk-women
9. Greco R, Noone B. Evidence-based medicine: reduction mammaplasty. Plast Reconstr Surg. 2017;139(1):230e. doi: 10.1097/PRS.0000000000002856
10. Perdikis G, Dillingham C, Boukovalas S, et al. American Society of Plastic Surgeons evidence-based clinical practice guideline revision: reduction mammaplasty. Plast Reconstr Surg. 2022;149(3):392e. doi:10.1097/PRS.0000000000008860
11. Merkkola-von Schantz PA, Kauhanen SMC, Jahkola TA, Krogerus LA, Hukkinen KS. Breast cancer detection by preoperative imaging in reduction mammaplasty patients: a single center study of 918 patients. World J Surg. 2017;41(8):2013-2019. doi:10.1007/s00268-017-3920-z
12. Usón Junior PLS, Callegaro Filho D, Bugano DDG, et al. Incidental findings in reduction mammoplasty specimens in patients with no prior history of breast cancer. An analysis of 783 specimens. Pathol Oncol Res. 2018;24(1):95-99. doi:10.1007/s12253-017-0230-6
13. Hassan FE, Pacifico MD. Should we be analysing breast reduction specimens? A systematic analysis of over 1,000 consecutive cases. Aesthetic Plast Surg. 2012;36(5):1105-1113. doi:10.1007/s00266-012-9919-9
14. Titley OG, Armstrong AP, Christie JL, Fatah MF. Pathological findings in breast reduction surgery. Br J Plast Surg. 1996;49(7):447-451. doi:10.1016/s0007-1226(96)90028-4
15. Nomikos A, Husain EA, Graham AD. Occult disease in reduction mammoplasties and prophylactic mastectomies. Breast J. 2020;26(4):691-696. doi:10.1111/tbj.13512
16. Ambaye AB, Goodwin AJ, MacLennan SE, Naud S, Weaver DL. Recommendations for pathologic evaluation of reduction mammoplasty specimens: a prospective study with systematic tissue sampling. Arch Pathol Lab Med. 2017;141(11):1523-1528. doi:10.5858/arpa.2016-0492-OA
17. Desouki MM, Li Z, Hameed O, Fadare O, Zhao C. Incidental atypical proliferative lesions in reduction mammoplasty specimens: analysis of 2498 cases from 2 tertiary women’s health centers. Hum Pathol. 2013;44(9):1877-1881. doi:10.1016/j.humpath.2013.02.015
18. Celik B, Senen Demiroz D, Yaz M, Muslu U. Radiologically innocuous breast reduction specimens. Should we send them to pathology lab anyway? G Chir. 2013;34(11-12):302-306.
19. Campbell MJ, Clark CJ, Paige KT. The role of preoperative mammography in women considering reduction mammoplasty: a single institution review of 207 patients. Am J Surg. 2010;199(5):636-640. doi:10.1016/j.amjsurg.2010.01.011
20. Colwell AS, Kukreja J, Breuing KH, Lester S, Orgill DP. Occult breast carcinoma in reduction mammaplasty specimens: 14-year experience. Plast Reconstr Surg. 2004;113(7):1984-1988. doi:10.1097/01.prs.0000122212.37703.6e
21. Noorbakhsh S, Koenig ZA, Hewitt N, et al. Atypical hyperplasia found incidentally during routine breast reduction mammoplasty: incidence and management. Plast Reconstr Surg Glob Open. 2022;10(2):e4141. doi:10.1097/GOX.0000000000004141
22. Bas S, Oner C, Aydin AC, Ucak R, Sirvan SS, Karsidag S. Discussion of histopathological findings of 954 breast reduction specimens. Sisli Etfal Hastan Tip Bul. 2021;55(1):42-48. doi:10.14744/SEMB.2020.33349
23. Fisher M, Burshtein AL, Burshtein JG, Manolas P, Glasberg SB. Pathology examination of breast reduction specimens: dispelling the myth. Plast Reconstr Surg Glob Open. 2020;8(11):e3256. doi:10.1097/GOX.0000000000003256
24. Huysmans M, Bronckaers M, Schillebeeckx C, Servaes D. Incidental breast carcinoma in reduction mammoplasty. Acta Chir Belg. 2017;117(5):308-311. doi:10.1080/00015458.2017.1328767
25. Pitanguy I, Torres E, Salgado F, Pires Viana GA. Breast pathology and reduction mammaplasty. Plast Reconstr Surg. 2005;115(3):729-734; discussion 735. doi:10.1097/01.PRS.0000152683.62899.50
26. Freedman BC, Smith SMR, Estabrook A, Balderacchi J, Tartter PI. Incidence of occult carcinoma and high-risk lesions in mammaplasty specimens. Int J Breast Cancer. 2012;2012:145630. doi:10.1155/2012/145630
27. Merkkola-von Schantz PA, Jahkola TA, Krogerus LA, Hukkinen KS, Kauhanen SM. Should we routinely analyze reduction mammaplasty specimens? J Plast Reconstr Aesthet Surg. 2017;70(2):196-202. doi:10.1016/j.bjps.2016.10.010
28. Tadler M, Vlastos G, Pelte MF, et al. Breast lesions in reduction mammaplasty specimens: a histopathological pattern in 534 patients. Br J Cancer. 2014;110(3):788-791. doi:10.1038/bjc.2013.708
29. Sofianos C, Zinn RJ, Geoffreys DA, Kruger D. Pathological findings in reduction mammoplasty specimens: a South African perspective.
S Afr Med J. 2015;105(4):308-311. doi:10.7196/samj.9108
30. Clark CJ, Whang S, Paige KT. Incidence of precancerous lesions in breast reduction tissue: a pathologic review of 562 consecutive patients. Plast Reconstr Surg. 2009;124(4):1033-1039. doi:10.1097/PRS.0b013e3181b45801
31. Klement KA, Hijjawi JB, Neuner J, Kelley K, Kong AL. Discussion of preoperative mammography in women undergoing reduction mammaplasty. Breast J. 2019;25(3):439-443. doi:10.1111/tbj.13237
32. Kuehlmann B, Vogl FD, Kempny T, et al. Occult pathologic findings in reduction mammaplasty in 5781 patients-an international multicenter study. J Clin Med. 2020;9(7):2223. doi:10.3390/jcm9072223
33. Berry DA, Cronin KA, Plevritis SK, et al; Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784-1792. doi:10.1056/NEJMoa050518
34. Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19 Suppl 1:14-25. doi:10.1258/jms.2012.012078
35. Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;2013(6):CD001877. doi:10.1002/14651858.CD001877.pub5
36. Tosteson ANA, Fryback DG, Hammond CS, et al. Consequences of false-positive screening mammograms. JAMA Intern Med. 2014;174(6):954-961. doi:10.1001/jamainternmed.2014.981
37. Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155(8):481-492. doi:10.7326/0003-4819-155-8-201110180-00004
38. Kalliainen LK; ASPS Health Policy Committee. ASPS clinical practice guideline summary on reduction mammaplasty. Plast Reconstr Surg. 2012;130(4):785-789. doi:10.1097/PRS.0b013e318262f0c0
39. Srivastava V, Meena RK, Ansari MA, Kumar D, Kumar A. A study of anxiety and depression in benign breast disease. J Midlife Health. 2020;11(4):200-209. doi:10.4103/jmh.JMH_85_20
40. Ren W, Chen M, Qiao Y, Zhao F. Global guidelines for breast cancer screening: a systematic review. Breast. 2022;64:85-99. doi:10.1016/j.breast.2022.04.003