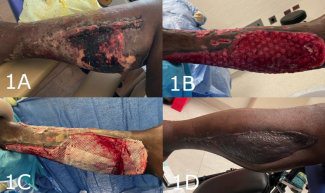
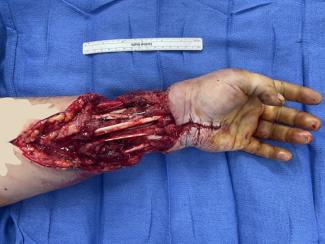
No Increase in Complications With Intravenous Tranexamic Acid Use in Vaginoplasty: A Retrospective Study
Abstract
Background. Across surgical specialties, tranexamic acid (TXA) is applied to reduce intraoperative and postoperative bleeding. Within plastic surgery, both topical and intravenous routes are used. The application of TXA has yet to be examined in vaginoplasties.
Methods. The authors performed a retrospective chart review of Mayo Clinic patients receiving penile inversion vaginoplasty from January 2017 through July 2021. Incidence of hematoma formation was assessed as the primary outcome. Secondary outcomes included perioperative hemoglobin, vaginoplasty complications, and possible TXA complications. These outcomes were compared across topical only (t-TXA), any intravenous (IV-TXA), and no TXA groups.
Results. Of the 124 vaginoplasties, 21 patients received t-TXA only and 43 received any IV-TXA. Only 4 patients developed a hematoma; 2 were from the no TXA group and 2 were from the any IV-TXA group. There was no significant change in perioperative hemoglobin across groups. Analysis showed lower incidence of divergent urine stream (odds ratio [OR], 0.499 [95% confidence interval (CI)], 0.316-0.789], P = .003) and neovaginal stenosis (OR, 0.435 [95% CI, 0.259-0.731], P = .002) within the any IV-TXA group and no increased incidence of other complications.
Conclusions. The use of either t-TXA or IV-TXA in vaginoplasty cases did not result in an increased rate of complications. There was no significant reduction in hematoma formation or postoperative hemoglobin decrease across groups.
Introduction
As part of gender-affirming care, some transwomen may choose to pursue vaginoplasty. The mainstay of this procedure includes orchiectomy, labioplasty, urethroplasty, and clitoroplasty.1 Some patients may also desire creation of a vaginal canal. One approach is penile inversion to create the introitus and proximal canal, with possible grafting from donor sites, such as the scrotal skin or medial thigh, to achieve sufficient depth.2 The creation of the canal is in a nonanatomic plane, and there are risks of hematoma formation. In addition, shearing and moisture in the perineal region make the surgery prone to graft failure.3,4
Effective intraoperative hemostasis is important in reducing ongoing blood loss. In addition to technical steps, another recent tool empirically applied to limit intraoperative and postoperative bleeding is tranexamic acid (TXA). TXA is an antifibrinolytic, which only carries formal US Food and Drug Administration approval for procedural prophylaxis in hemophilia patients and treatment of menorrhagia.5 Its use has been extrapolated to many surgical applications. Studies have shown the effective use of the intravenous-TXA (IV-TXA) formulations in reducing transfusion requirements and drain output in total joint arthroplasties.6,7 Outside of orthopedics, TXA’s usage has been examined within trauma,8,9 neurosurgery,10 and obstetrics practice,11 among others with results suggesting or demonstrating the treatment’s efficacy.
Within the plastic surgery domain, TXA is also often administered topically. Among survey respondents within the American Society of Plastic Surgeons, around 18% used TXA routinely in their practice; of these surgeons, nearly half gave TXA either topically alone or in combination with an IV bolus.12 Topical-TXA (t-TXA) in randomized controlled trials has been found to reduce operative blood loss as well as the need for blood transfusion when compared with placebo or no TXA use.13 Within reconstruction applications, t-TXA has demonstrated efficacy in reducing drain output in mastectomy14 and reduction mammoplasty.15 For those utilizing the liposuction technique in mammoplasty reduction, the addition of t-TXA, as a component of Klein’s solution, with IV-TXA administration decreased liposuction decanted volume and dermal bleeding during de-epithelialization when compared with those procedures that did not employ this local component.16 Moreover, both single and dual-route administration of this agent have reduced intraoperative and postoperative blood loss or drainage in various aesthetic surgeries, although the clinical significance of this reduction remains unclear.
Although TXA’s application has been extended to vaginoplasties from the other specialty and plastic surgery reconstructive procedures, the authors are unaware of studies examining the efficacy of either route or of combined administration in vaginoplasties. Thus, a retrospective review of vaginoplasty cases was performed at their institution to characterize the route of TXA administration and to compare outcomes for these routes.
Methods and Materials
Patient Cohort
A retrospective case series review was performed for patients who underwent penile inversion vaginoplasty at Mayo Clinic from January 2017 through July 2021. Patients were excluded if they withdrew their Minnesota (MN) research authorization.
Institutional Practice
Over this time frame, 3 primary surgeons performed vaginoplasties. Additionally, TXA usage was not introduced into practice at the institution until 2019. The t-TXA formulation usually includes 3 g of TXA in 75 µL of saline, whereas IV-TXA is administered as a 1-g IV bolus at the beginning of the procedure.
Data Collection
Patient demographic information and comorbidities at the time of surgery were recorded including age; body mass index; smoking status; and diagnosis of hypertension, chronic obstructive pulmonary disease (COPD) or asthma, cardiovascular disease, and diabetes. Intraoperative use of TXA (topical and IV) was recorded.
To assess the efficacy of these interventions, the primary outcome, hematoma formation, was recorded. The secondary outcome of difference in hemoglobin (postoperative minus preoperative), when available, was also recorded, and other surgical complications, such as graft loss, neovaginal stenosis, or dehiscence, were documented. Multiple complications were classified as minor or major depending on provider follow-up notes; such descriptors as mild or moderate warranted minor classification, whereas complications characterized as substantial, severe, or functionally impairing were classified as major. Additionally, possible adverse outcomes of TXA were recorded as well as the patient’s final follow-up date.
Statistical Analysis
Patients were stratified into the following groups based on TXA usage and route: no TXA, t-TXA only, and any IV-TXA use (IV-TXA alone or both IV- and t-TXA). Statistical analysis was performed using Excel (Microsoft Corp) and SPSS v26 (IBM Corp) software. Significance was assessed for parametric and nonparametric data using ANOVA (analysis of variance) and Kruskal-Wallis testing, respectively. The association between TXA use and complications was evaluated by univariate and multivariate analysis, and the odds ratio (OR) with 95% confidence interval (CI) and P values were calculated using Cox regression or logistic regression as appropriate. All tests were 2-sided, and a P value of <.05 was considered statistically significant.
This study was approved as exempt by the Mayo Clinic Institutional Review Board in Rochester, MN.
Results
Overview
A total of 124 patients who underwent penile inversion vaginoplasty were included in this review. An overview of number of cases and complications (major and minor) over this time frame is shown in Table 1.

Patient Cohort
T-TXA alone, IV-TXA alone, and the combination of t-TXA and IV-TXA were used in 21 (16.9%), 2 (1.6%), and 41 (33.1%) cases, respectively. Furthermore, the cohort of any IV-TXA use included 43 patients. The remaining 60 patients (48.4%) received no TXA formulation. Demographic data and comorbidities for these groups are shown in Table 2. Cohort characteristics were not significantly different except for the higher incidence of active smoking within the no TXA group, former smoking within the any IV-TXA use group, and COPD/asthma within the only t-TXA group. The average length of time to last postoperative follow-up was 34.0 weeks.

Hematoma Formation
Concerning the primary outcome, only 4 patients (3.2%) developed a hematoma, one of which was classified as major. The major hematoma formed in a patient who did not receive TXA. The other 3 hematomas were classified as minor. While one of these occurred within the no TXA group, the other 2 minor hematomas affected patients receiving both t- and IV-TXA.
The major hematoma was located at the neovaginal introitus, whereas the minor ones were identified along the labia or mons pubis. The major hematoma required return to the operating room for evacuation. Minor hematomas warranted observation, drainage, or aspiration in clinic.
Perioperative Hemoglobin
Preoperative and postoperative hemoglobin measurements were available for 77 patients. Overall, the average difference in hemoglobin was a decrease in 1.8 g/dL. Among these patients, 15 received t-TXA alone (average difference, -1.2), 31 received IV-TXA (average difference, -1.9), and 31 did not receive TXA (average difference, -2.1). Comparison of these groups did not show these differences to be significant (P = .150).
Other Vaginoplasty Complications
Overall, only 6 (4.8%) vaginoplasty cases had no complications, although few patients (17, 13.7%) had major complications. The most common major complications included wound dehiscence (2.4%) and infection (2.4%). Likewise, wound dehiscence (46.7%) and tissue necrosis (30.6%) were the most frequent minor complications. Additional complications not categorized by severity included granulation tissue, divergent urine stream, and neovaginal stenosis affecting of 76, 28, and 24 patients, respectively. Notably, no patients developed a fistula or an adverse thromboembolic event (VTE) from the TXA.
Across this population, on average, patients experienced 2.2 complications. This complication frequency was similar among the subgroups (shown in Table 3). While rates of various complications were similar across subgroups, analysis showed significantly lower incidence of divergent urine stream (OR, 0.499 [95% CI, 0.316-0.789], P =.003) and neovaginal stenosis (OR, 0.435 [95% CI, 0.259-0.731], P =.002) within the any IV-TXA group. Additionally, several confounding factors were investigated using univariate and multivariate analysis, including the effect of smoking and the difference in surgeon’s experience and skills over time, and no statistically significant differences across the groups were found.

Discussion
Overall, there was no significant reduction in hematoma development between the use of IV-TXA or only t-TXA groups. Additionally, neither group showed measurable improvement in this outcome when compared with those patients who did not receive TXA. Although efficacy of these interventions for the primary outcome was not found in this retrospective case series, the results demonstrated no increased adverse events or complications with either TXA route in vaginoplasty, suggesting it is a safe agent to use in these procedures.
Overall, hematomas complicated 3.2% of cases. This finding is consistent with other studies reporting hematoma formation in 4% to 6% of vaginoplasties.17 In the authors’ operative experience, the primary source of bleeding arises during the canal dissection from the bleeding veins near the urethral bulb. Anecdotally, use of TXA offered better visualization of critical structures during dissection through reduced bleeding and need for electrocautery coagulation. This improvement was particularly noticed during canal dissection. This better visualization may improve safety, although there were no complications related to canal dissection, such as fistula, to demonstrate this hypothesis. Standard protocols for hematoma prevention include no use of chemoprophylaxis and use of bulky bolster and stent in the neovaginal canal until postoperative day 4. In addition to TXA use, these interventions may also be critical in preventing hematoma formation. Although the efficacy of TXA in reducing hematoma formation in vaginoplasties was not demonstrated in this review, its effectiveness in other surgeries6,14,15 suggests it may reduce hematomas or other intra- or postoperative bleeding that can have clinically significant sequelae.
If untreated, hematomas can precipitate tissue necrosis, partial/total graft loss, and fistula formation.3 These complications were seen in 32.3%, 21.8%, and 0% of patients, respectively, in this retrospective analysis. At the authors’ institution, efforts to maintain graft adherence and prevent fluid accumulation include placing a vaginal stent condom and packing the vaginal cavity with antibiotic impregnated and bulky bolster dressing to reduce incidence of hematoma formation causing graft loss. Chemoprophylaxis is not used, and patient ambulation is encouraged from postoperative day 0. In this review, only 1 hematoma was located at the neovaginal introitus near the graft. This patient also had major graft loss, although location of graft loss was not examined. Significant decrease in divergent urinary stream may be a product of other confounding variables not examined, such as surgical technique of urethral meatus creation and obesity. Likewise, the significant reduction in vaginal stenosis may be the result of other postoperative complications, such as poor graft take and infection, or issues with postoperative care, such as inconsistent canal dilation.
In this study, the average perioperative change in hemoglobin was not significant between groups; the overall average decrease was 1.8 units. Whereas volume status (with resuscitation dilution or insensible loss hemoconcentration) can impact the accuracy of this change, these measurements have been used in prior studies to validate TXA efficacy. Still, if not warranting transfusion, the clinical significance of a postoperative decrease remains unclear. Thus, the absence of significant improvement in perioperative hemoglobin concentration in patients who received TXA need not restrict practice and intervention recommendations for TXA in vaginoplasty.
Notably, no patients experienced VTE with TXA use. VTE incidence may also be impacted by the ambulation protocol beginning on postoperative day 0. Additionally, this complication has been a theoretical concern of IV-TXA; however, studies have not found an association between IV-TXA and VTE incidence.18 The use of t-TXA has still been strongly considered given its similar efficacy to the IV formulation and potential mitigation of theoretical VTE risk and other side effects through decreased systemic absorption.19 Reports in the literature have shown an association between TXA usage and an increased frequency of seizures in the postoperative period.20 When deciding on TXA use in vaginoplasty, this seizure association should be considered along with other predisposing patient factors.
Limitations
Although this review included a small cohort of vaginoplasty cases, the authors were reassured to see no increase in complications with any routes of TXA administration. There was limited incidence of the primary outcome, making it challenging to find significance in TXA efficacy for either group. Likewise, not all patients had perioperative hemoglobin measurements reported, which restricted assessment of this outcome as well. While other short- and long-term complications were tracked, multiple factors may contribute to their development, making a reduction in complication rates difficult to attribute to the benefits of TXA use.
Future studies would benefit from a randomized trial approach comparing the 2 routes of TXA, topical and IV. This would address issues with surgeon turnover or other confounding factors, allowing for more effective assessment of whether TXA does reduce intraoperative bleeding or hematoma development. Additionally, future studies would benefit from a third arm in which patients received TXA via both topical and IV routes to see if dual-route application offers additional benefits or risks (the current study’s cohort of patients receiving IV-TXA only was too small for separate analysis). The assessment of drain output, blood transfusion requirements, and perioperative hemoglobin concentrations can serve as additional metrics in assessing the efficacy of the intervention, given the low incidence of hematoma formation in cases. Also, since TXA can provide benefit for intraoperative visualization, surgeons could score the difficulty of cases from a bleeding perspective. Further, as studies within other fields have suggested, the optimal administration timing and dosage for any TXA routes still need to be elucidated in future trials.
Conclusions
The use of either or both t-TXA and IV-TXA in penile inversion vaginoplasties was not associated with increased complications in this case series. There was no reduction in hematoma formation or postoperative hemoglobin drop based on use or route of TXA. In the authors’ experience, TXA helps with intraoperative visualization. Based on these results, TXA is a tool that can be safely used in vaginoplasty, depending on surgeon preference.
Acknowledgments
Affiliations: 1Division of Plastic and Reconstructive Surgery, Mayo Clinic, Rochester, MN; 2Mayo Clinic Alix School of Medicine, Mayo Clinic, Rochester, MN; 3Department of Urology, Mayo Clinic, Rochester, MN
Correspondence: Thanapoom Boonipat, MD; boonipat.thanapoom@mayo.edu
Ethics: IRB: Toward an Objective Assessment of Surgical Outcomes and Facial and Body Deformity Using Eyetracking Technology; ID 17-009087
Funding: No funding was received for this study.
Disclosures: The authors disclose no relevant conflict of interest or financial disclosures for this manuscript.
References
1. Pariser JJ, Kim N. Transgender vaginoplasty: techniques and outcomes. Transl Androl Urol. Jun 2019;8(3):241-247. doi:10.21037/tau.2019.06.03
2. Shoureshi P, Dugi D, 3rd. Penile Inversion Vaginoplasty Technique. Urol Clin North Am. Nov 2019;46(4):511-525. doi:10.1016/j.ucl.2019.07.006
3. Ferrando CA. Vaginoplasty Complications. Clin Plast Surg. Jul 2018;45(3):361-368. doi:10.1016/j.cps.2018.03.007
4. Dreher PC, Edwards D, Hager S, et al. Complications of the neovagina in male-to-female transgender surgery: A systematic review and meta-analysis with discussion of management. Clin Anat. Mar 2018;31(2):191-199. doi:10.1002/ca.23001
5. Chauncey JM, Wieters JS. Tranexamic Acid. StatPearls. StatPearls Publishing
StatPearls Publishing LLC; 2022.
6. Seol YJ, Seon JK, Lee SH, et al. Effect of tranexamic acid on blood loss and blood transfusion reduction after total knee arthroplasty. Knee Surg Relat Res. Sep 2016;28(3):188-93. doi:10.5792/ksrr.2016.28.3.188
7. Castro-Menéndez M, Pena-Paz S, Rocha-García F, Rodríguez-Casas N, Huici-Izco R, Montero-Viéites A. Efficacy of 2 grammes of intravenous transexamic acid in the reduction of post-surgical bleeding after total hip and knee replacement. Rev Esp Cir Ortop Traumatol. Sep-Oct 2016;60(5):315-324. doi:10.1016/j.recot.2016.05.001
8. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. Feb 2012;147(2):113-9. doi:10.1001/archsurg.2011.287
9. Perel P, Al-Shahi Salman R, Kawahara T, et al. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury--a nested randomised, placebo-controlled trial. Health Technol Assess. 2012;16(13):iii-xii, 1-54. doi:10.3310/hta16130
10. Hansen JK, Lydick AM, Wyatt MM, Andrews BT. Reducing postoperative bleeding after craniosynostosis repair utilizing a low-dose transexamic acid infusion protocol. J Craniofac Surg. Jul 2017;28(5):1255-1259. doi:10.1097/scs.0000000000003711
11. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. May 27 2017;389(10084):2105-2116. doi:10.1016/s0140-6736(17)30638-4
12. Brown SMB, Tal; Taub, Peter MD; Rohrich, Rod MD, FACS. The role of tranexamic acid in plastic and reconstructive surgery: a national perspective. Plast Reconstr Surg Glob Open. 2021;9(10S):21. doi:10.1097/01.GOX.0000799192.39058.b8
13. Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev. Jul 23 2013;(7):Cd010562. doi:10.1002/14651858.CD010562.pub2
14. Ausen K, Hagen AI, Østbyhaug HS, et al. Topical moistening of mastectomy wounds with diluted tranexamic acid to reduce bleeding: randomized clinical trial. BJS Open. Apr 2020;4(2):216-224. doi:10.1002/bjs5.50248
15. Ausen K, Fossmark R, Spigset O, Pleym H. Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. Br J Surg. Oct 2015;102(11):1348-1353. doi:10.1002/bjs.9878
16. Abboud NM, Kapila AK, Abboud S, Yaacoub E, Abboud MH. The combined effect of intravenous and topical tranexamic acid in liposuction: a randomized double-blinded controlled trial. Aesthet Surg J Open Forum. Jan 2021;3(1):ojab002. doi:10.1093/asjof/ojab002
17. Horbach SE, Bouman MB, Smit JM, Özer M, Buncamper ME, Mullender MG. Outcome of vaginoplasty in male-to-female transgenders: a systematic review of surgical techniques. J Sex Med. Jun 2015;12(6):1499-1512. doi:10.1111/jsm.12868
18. Taeuber I, Weibel S, Herrmann E, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis, and meta-regression. JAMA Surg. Apr 14 2021;156(6):e210884. doi:10.1001/jamasurg.2021.0884
19. Montroy J, Hutton B, Moodley P, et al. The efficacy and safety of topical tranexamic acid: a systematic review and meta-analysis. Transfus Med Rev. Feb 19 2018;S0887-7963(17)30151-30157. doi:10.1016/j.tmrv.2018.02.003
20. Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: causes and treatment. Ann Neurol. Jan 2016;79(1):18-26. doi:10.1002/ana.24558