Use of Suprapubic Panniculus for Split-Thickness Skin Graft in Buried Penis Repair
© 2023 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. In the United States, acquired buried penis deformity is an increasingly more common condition. Management of the buried penis deformity is accomplished with removal of macerated skin and subcutaneous tissue from the panniculus and prepubic region, and replacement of denuded penile skin. If local tissue advancement is insufficient to cover the defect, a skin graft may be required. Though the anterior thigh is commonly used, this creates a second defect. Here we describe 2 cases of split-thickness skin grafts harvested from the panniculus to cover buried penis deformities.
Methods. Two patients with a buried penis deformity were identified. The denuded suprapubic tissue was elevated. Using inferior traction, split-thickness skin grafts were harvested and placed onto the shaft of the penis. The remaining excess tissue was resected.
Results. One patient had a fungal rash that resolved with topical treatment. The other patient had a hematoma requiring surgical evacuation. Neither patient had any other complications, and both had over 95% take of the split-thickness skin grafts.
Conclusions. These cases demonstrate the successful use of pannicular skin grafts for buried penis deformity correction. This donor site avoids creation of a second defect. As demonstrated here, the grafts are a durable option, even in the setting of local infection and hematoma.
Introduction
Obesity rates in the United States have increased over the last 2 decades, with a concomitant rise in associated medical conditions, including hypertension, type 2 diabetes, and in men, acquired buried penis deformity.1 A buried penis deformity most often results from overhanging prepubic skin and fat but can also be caused by skin deficiencies, Fournier’s gangrene, and localized hidradenitis.2 Continuous pressure, urine pooling, and moisture trapping from the soft tissue results in increased inflammation, skin breakdown, infections, and scar tissue formation. Eventually, this can reduce penile mobility and result in penile retraction.3 In addition to cosmetic concerns, the patient may also have difficulty with hygiene, voiding, or sexual function.2
Management of the buried penis deformity is accomplished with removal of redundant macerated skin and subcutaneous tissue from the panniculus and prepubic region, and replacement of denuded penile skin after releasing contracted scar tissue. If the patient has enough mobility in the remaining healthy penile skin, the defect can be repositioned ventrally and covered with a scrotal Z-plasty flap.3 However, in patients with phimotic scar tissue that prevents mobilization or extensive skin breakdown, there is likely to be a larger defect requiring a skin graft. Though the anterior thigh is a commonly used donor site for split-thickness skin grafts, there is an associated risk of donor site morbidity. An alternative donor site that avoids creation of a second defect is the panniculus. This tissue is regularly discarded, but portions may be used for skin grafts. Here, we report on 2 cases of buried penis reconstruction with split-thickness skin grafts adequately harvested from the panniculus.
Methods
This human subjects research was approved by theTulane University Institutional Review Board (IRB #2022-1714).
Patient 1
Patient 1 (BMI 41.64) first presented to urology with a urinary tract infection and prostatitis requiring intravenous antibiotics. He was diagnosed with lichen sclerosis, buried penis deformity, and pan-urethral stricture. Per his urologist’s recommendation, his initial operation was a urethroplasty completed 6 months prior, then a second surgery was planned to manage the buried penis and lichen sclerosis.
Patient 2
Patient 2 (BMI 40.19) came to the urology clinic following an emergency room visit for urinary retention, at which time a Foley catheter was placed. This patient initially weighed over 400 lb but had lost approximately 100 lb, resulting in significant excess skin that caused damage to the prepubic region. He was diagnosed with a buried penis deformity, and surgical repair was planned.


Surgical technique. First, the urologic surgeon released the penile cicatrix, inserted a 16 French Foley catheter, and degloved the remaining unhealthy epithelium off the penis (approximately 90% of the penis in the first case, approximately 100% in the second case). At this stage, a W-shaped or trapezoid-shaped suprapubic lipectomy was marked (Figure 1). The markings of the incisions were made from 1 cm below the infra-pannicular crease or waistline sulcus to 3 cm proximal to the penile base. The inferior and lateral incisions were made and sharp dissection occurred down to the rectus fascia. The dissection was then carried out cephalad to the extent of our resection. A region of skin atop the area to be resected was identified. Before the superior division was made, penetrating towel clamps were fastened to the caudal edge of the flap. Traction was applied inferiorly to pull the tissue taut. This counteracted the laxity and irregularity of the suprapubic tissue, allowing it to be a flat, suitable surface to harvest skin (Figure 2). A 0.5-mm-thick split-thickness skin grafts (STSG) was harvested using a dermatome (112 cm2 in patient 1, 110 cm2 in patient 2). The resection of the elevated tissue was then completed by making the superior incisions and dividing the excess tissue. Two 15 Blake drains were placed, and the abdominal incision was closed in 3 layers with a layer of progressive tension quilting sutures as the first layer to decrease incision closure tension and minimize dead space. Then, any excess tissue was excised from the proximal scrotum (12 cm2 in the first patient), and a Z-plasty closure was performed.
The skin graft was either meshed or manually fenestrated in a 1-to-1.5 ratio. It was then placed onto the shaft of the penis without excessive spreading of the interstices, and finally sutured in place with 4-0 Chromic sutures used around the proximity and as bolstering sutures (Figure 3, Figure 4). The STSG was covered with an extracellular matrix graft dressing and a fibrin sealant to promote healing and graft adherence, and a silver silicone nonadherent dressing was placed on top. Bolster dressings were used to cover the penis, scrotum, and abdominal incision, and held in place with mesh underwear.


Results
Patient 1
The patient was kept overnight for observation and discharged home the following day. In the hospital, the patient received scheduled acetaminophen, with oral oxycodone as needed and intravenous hydrocodone for breakthrough pain. Following discharge, his postoperative regimen included oxybutynin for 60 days, a 2-week course of sulfamethoxazole-trimethoprim, and acetaminophen and nonsteroidal anti-inflammatory drugs as needed for pain management. The abdominal drains were removed when the drainage decreased to less than 30 cc per day, and the Foley catheter was kept in place for 4 weeks due to history of urethral spasms following initial urethroplasty.

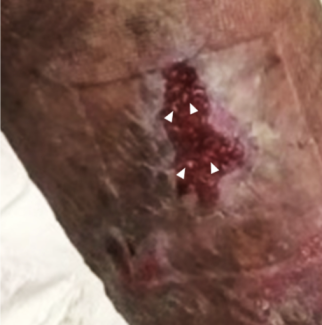
At the patient's 1-month follow-up visit, his STSG had over 95% take (Figure 5), and he was not experiencing any urinary symptoms. The patient had a fungal rash under his abdominal pannus prior to surgery. This persisted after surgery but resolved with twice-daily application of nystatin powder. Patient interviews at each follow-up appointment reported continuing satisfaction with outcomes. He also reported partial return of sensation to his graft recipient site over the course of follow up. The patient was allowed to resume sexual activity after his follow-up appointment at 2 months to ensure appropriate time for penile healing and graft take, and the urological team continued to follow the patient’s improvement in sexual function.
Patient 2
This patient stayed in the hospital 5 days postoperatively. On day 2, he had a small subcutaneous abdominal hematoma that was evacuated at the bedside. The skin graft on the penile shaft was unaffected. The patient completed a 1-week course of ciprofloxacin, and 5 days of ketoconazole with prednisone. Inpatient pain control included scheduled acetaminophen and oxycodone as needed, then the patient continued to use acetaminophen as needed following discharge. The abdominal drains were removed on day 11 when drainage had decreased to less than 30 cc per day, and the Foley catheter was removed at 7 days.

At his 1-month follow-up visit, the patient had successful take of his STSG (over 95%, Figure 6) and resolution of his preoperative urinary retention. The remaining penile wounds healed with outpatient wound dressing changes. There was no recurrence of his hematoma and, most importantly, he did not have any other postoperative complications involving the skin graft. Patient satisfaction was assessed with patient interviews at each follow-up appointment, where he reported continuing satisfaction with outcomes. He also reported that sensation increased over his graft recipient site over the course of follow-up. The patient was allowed to resume sexual activity after following up at 2 months to ensure appropriate time for penile healing and graft take, and the urological team continued to follow the patient’s improvement in sexual function.
Discussion
Correction of the buried penis deformity is challenging for several reasons. First, the condition is often seen in patients that are morbidly obese, like in the cases reported above. Ideally, patient optimization prior to surgery involves management of weight. However, in some patients, this is not possible or practical. Patient 2 lost approximately 100 lb prior to his operation but still had a BMI above 40. Thus, patient optimization should involve evaluation and management of other comorbidities prior to operation. In both our patients, a thorough preoperative assessment was conducted to minimize perioperative risk.
Second, patients often have urological complications associated with their buried penis deformity. In our practice, these patients are most often referred to us by urology and thus have ongoing urological care. Among all patients with buried penis deformity, preoperative evaluation by a urologist is important to ensure complete management of any urinary concerns.
Another challenge in correcting the buried penis deformity is identifying a suitable skin graft for the penis. There are several requirements for a penile skin graft. Ideally, it should be hairless, have sufficient elasticity to allow for erections, and experience minimal contracture to avoid recurrence of phimosis or retraction.4 A full-thickness skin graft is more elastic and results in less secondary contracture; however, it may carry hair follicles, requires closure of the donor site, and may suffer from decreased skin graft take rate. The STSG has greater secondary contracture but is harvested without hair follicles, can be meshed to provide greater coverage, and may be a better aesthetic match for thin penile skin.5 Therefore, we used STSGs and draped the graft without excess tension with minimal expansion of the graft interstices to minimize secondary contracture.
Various donor sites can be used for the penile skin graft. While the anterior thigh is a common choice,4-6 it creates a visible defect that is at risk for unsightly scarring or contour irregularities, numbness, irregular pigmentation, pruritis, and bleeding. In addition, the patient often experiences pain from the donor site. Instead, we chose to harvest the skin graft from the discarded panniculus, which eliminates donor site concerns. Because the inferior surface is often adjacent to macerated skin due to contact with the caudal pubic region, there is often reluctance to harvest this tissue. Ideally, donor site tissue for STSG is completely healthy and free from disease. In patients with sizeable defects or widespread intertrigo, finding a large enough donor site from the resectable tissue can be seen as a challenge. Meshing the STSG allows for the use of smaller grafts, but the graft must be adequately sized to cover the penis while erect. In these two cases, there was enough excess skin in the panniculus to use a meshed STSG without stretching the interstices excessively. Additionally, our grafts from the panniculus have shown to be a durable option in the setting of local infection or local hematoma.
Overall, both patients described here had improvement in quality of life postoperatively and suffered minimal complications. Successful use of the panniculus for the penile skin graft has been described only few times in the literature 5,7,8, and this case series adds to the body of evidence supporting its use as a reliable and trustworthy option.
Acknowledgments
Affiliations: 1Tulane University School of Medicine, New Orleans, Louisiana; 2Division of Plastic and Reconstructive Surgery, Department of Surgery, Tulane University School of Medicine, New Orleans, Louisiana
Correspondence: Abigail E. Chaffin, MD, FACS, CWSP, MAPWCA; achaffin@tulane.edu
Ethics: This human subjects research was approved by theTulane University Institutional Review Board (IRB #2022-1714).
Disclosures: The authors disclose no relevant financial or nonfinancial interests.
References
1. Centers for Disease Control and Prevention. Adult obesity facts. https://www.cdc.gov/obesity/data/adult.html
2. Ho TS, Gelman J. Evaluation and management of adult acquired buried penis. Transl Androl Urol. 2018;7(4):618-627. doi:10.21037/tau.2018.05.06
3. Flynn KJ, Vanni AJ, Breyer BN, Erickson BA. Adult-acquired buried penis classification and surgical management. Urol Clin North Am. 2022;49(3):479-493. doi: 10.1016/j.ucl.2022.04.009
4. Thakar HJ, Dugi DD, 3rd. Skin grafting of the penis. Urol Clin North Am. 2013;40(3):439-48. doi:10.1016/j.ucl.2013.04.004
5. Anandan L, Mohammed A. Surgical management of buried penis in adults. Cent Euro J Urol. 2018;71(3):346-352. doi:10.5173/ceju.2018.1676
6. Burns H GJ, Chowdhry S, Lee T, Schulz S, Wilhelmi BJ. Comprehensive review and case study on the management of buried penis syndrome and related panniculectomy. Eplasty 2018;18(e5).
7. Figler BD, Gan ZS, Mohan CS, Zhang Y, Filippou P. Outpatient panniculectomy and skin graft for adult buried penis. Urology. 2020;143:255-256. doi: 10.1016/j.urology.2020.04.129.
8. Monn MF, Socas J, Mellon MJ. The use of full thickness skin graft phalloplasty during adult acquired buried penis repair. Urology. 2019;129:223-227. doi: 10.1016/j.urology.2019.04.007.