Simultaneous Sentinel Lymph Node Biopsies Using Both the Magtrace/Sentimag System and Radioactive Isotope Tracer/Blue Dye Dual Technique for Concurrent Breast Carcinoma and Malignant Melanoma
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. Breast cancer and melanoma are extremely common, with a growing incidence in the United Kingdom. In this case report, we present a patient with synchronous melanoma and breast carcinoma, with focus on the simultaneous use of 2 sentinel lymph node biopsy mapping techniques.
Methods. The use of 2 mapping techniques in this case is necessary to ensure the accurate identification of the correct sentinel node (for each respective primary malignancy), providing vital prognostic information and allowing for appropriate adjuvant therapy. The report describes the use of a single surgical incision to access both melanoma and breast carcinoma sentinel lymph nodes.
Conclusions. The report highlights the technical possibility of using both the radioactive isotope tracer/blue dye dual technique and the Magtrace/Sentimag system without interference or complication.
Introduction
In this article, we report a case of a synchronous melanoma and breast carcinoma. Melanoma is a common skin malignancy, arising from the malignant transformation of melanocytes. Breast cancer and melanoma account for approximately 15% and 5%, respectively, of all new diagnosed cancer cases in the United Kingdom.1 Melanoma is typically managed by wide local excision (WLE) and sentinel lymph node biopsy (SLNB), and may require adjuvant immunotherapy or completion lymphadenectomy. SLNB refers to a procedure to sample the first draining lymph node (“sentinel node”) from the primary tumor and has been proven the most important prognostic factor in melanoma cases. Similarly, patients with breast cancer will undergo either WLE or mastectomy (depending on breast size, tumor size, and location) and SLNB, followed by adjuvant therapy. Biopsy-proven positive lymph nodes would routinely be discussed by the relevant multidisciplinary teams (MDTs) to ensure appropriate postoperative therapy – immunotherapy or targeted therapy in melanoma patients and chemotherapy or radiotherapy for breast cancer patients.
The conventional (standard practice) method of locating sentinel lymph nodes is using the radioactive isotope tracer and blue dye dual technique; this has a reported 97.7% identification rate in melanoma patients.2 The dual technique remains the gold standard in both breast and malignant melanoma.3 Accurate staging of melanoma and breast cancers is of utmost importance prognostically, and also because it allows access to adjuvant therapies. Therefore, when presented with 2 simultaneous primary tumors requiring SLNB, accurate identification of the correct draining node (for the specific primary tumor) becomes extremely difficult due to significant cross-contamination from the radioactive marker and blue dye. Therefore, in cases with dual diagnoses requiring mapping of the same lymphatic basin, alternative techniques and combinations allow identification of the sentinel node for each diagnosis.
Recently, the National Institute for Health and Care Excellence (NICE) guidance in breast cancer was updated to include the use of the Magtrace/Sentimag system for SLNB as an acceptable alternative to the radioactive tracer/blue dye dual technique.4 The Magtrace and Sentimag system comprises a magnetic liquid tracer (Magtrace), which is injected preoperatively into the primary tumor site, and a handheld magnetic sensing probe (Sentimag) to identify the sentinel node intraoperatively. This system is advantageous as it eliminates the radiation risk from the radioactive tracer and complications of the blue dye injection (such as anaphylaxis), but its use remains limited depending on the specific unit and is not currently utilized in melanoma patients.5
A literature search revealed 1 other case report of a patient diagnosed with simultaneous breast cancer and melanoma.6 However, there are no reports discussing the synchronous use of 2 different SLNB mapping techniques. This report focuses on our experience with the simultaneous use of 2 SLNB mapping techniques to accurately map the lymph node basins in a challenging dual-diagnosis case. The report describes the use of a single surgical incision to access both melanoma and breast carcinoma sentinel lymph nodes and highlights the technical possibility of using both the radioactive isotope tracer/blue dye dual technique and the Magtrace/Sentimag system without interference or complication. Written patient consent was obtained for the following case report.
Case Report
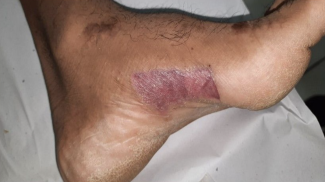
A 51-year-old woman, with no previous past medical history, was referred to dermatology in Autumn 2022 with a brown-black pigmented growth on her left index finger (LIF) that had been increasing in size during the previous 8 months (Figure 1). The lesion was initially noted by the patient after nailbed trauma. The patient was repeatedly treated for multiple nail infections with antibiotics, with no improvement. A biopsy (2 weeks post-referral) confirmed the diagnosis of a malignant melanoma (MM), triggering a referral to our tertiary plastic surgery unit.

Figure 1. A clinical photograph of the left index finger malignant melanoma (after blue dye injection), shown from the dorsal (A), volar (B), and straight on (C) views.
During this time, the patient presented to her primary physician with palpable 2-cm right-sided and 1-cm left-sided breast lumps. She was referred to the breast surgery team, and the subsequent triple assessments (nil palpable axilla lymphadenopathy), alongside a strong family history for breast cancer, were suggestive of a malignancy. Biopsy confirmed bilateral breast cancer; right invasive carcinoma of no special type (G3, ER 8/8 PgR 7/8 HER-2 negative) and left invasive ductal carcinoma (G2, ER 8/8, PgR 8/8, HER-2 borderline).
Joint care of the patient was arranged by the plastic and breast surgery teams. The patient was discussed at separate skin cancer and breast cancer MTDs. As per national guidelines for IIC melanoma, this patient underwent BRAF testing, a staging scan (demonstrating no metastatic spread of either the malignant melanoma or breast carcinomas), and required surgical removal and SLNB (no specific technique dictated in guidance).7 National breast cancer management guidelines recommended breast-conserving surgery and sentinel lymph node biopsy (no ultrasound-detected lymph node involvement).4
Under a single general anaesthetic, the plastic surgeons performed an amputation of the LIF at the proximal interphalangeal joint (for local disease control) and an ipsilateral axillary SLNB using the conventional dual technique (radioactive isotope tracer and blue dye). The breast surgeons performed a bilateral WLE of breast lumps with bilateral SLNBs using the Magtrace/Sentimag system.
Two weeks postoperatively, the histology was discussed at both the skin and breast MDTs. Complete excision with clear margins was achieved for both the breast cancer and melanoma. No metastatic spread was detected in the sentinel lymph nodes. The breast MDT recommended bilateral radiotherapy and tamoxifen hormone therapy (ER+ve). The patient had a high oncotype risk score, and therefore chemotherapy was also offered. The skin MDT recommended the use of immunotherapy with pembrolizumab (as per recent guidance on stage 2C melanoma). However, the patient declined any further adjuvant therapy at present. Three months later during routine follow-up, a left epitrochlear in-transit metastasis was palpated. The patient subsequently went on to have a completion lymphadenectomy of the axilla and excision of the in-transit disease. Currently, the patient is on adjuvant pembrolizumab and remains under follow-up with the plastic and breast surgery teams.
Discussion
This case demonstrates the use of a single incision for both SLNBs and successful retrieval of the respective sentinel lymph nodes for both the malignant melanoma and breast carcinoma (Figures 2-4). This approach, which has not been previously published in the literature, reduced the total number of surgical procedures needed, overall hospital stay, and possible anaesthetic and surgical complications. The diagnosis of aggressive melanoma and synchronous bilateral breast cancers (lobular and ductal) both warranted separate urgent surgical removal and evaluation of local lymph nodes; a single procedure ensured both malignancies were investigated in a timely manner.

Figure 2. A clinical photograph demonstrating the single incision approach to the axilla, allowing for identification of both sentinel lymph nodes.

Figure 3. Sentinel lymph node for the breast carcinoma.

Figure 4. Sentinel lymph node for the malignant melanoma.
In this case, the left-sided SLNBs were conducted using both the conventional radioactive isotope tracer/blue dye dual technique and Magtrace/Sentimag system. The 2 techniques worked well simultaneously, allowing for correct identification of the respective sentinel lymph nodes of both the malignant melanoma and breast carcinoma. No complications occurred in the intraoperative or postoperative period that were related to the mapping techniques. This case demonstrates the need for surgeons to be aware of the range of techniques currently available for SLNB, and although the radioactive tracer/blue dye dual technique remains the gold standard, safe and effective techniques (such as the Magtrace/Sentimag system) do exist as viable alternatives.8 Without the use of 2 mapping techniques, such as in the case, accurate staging of these cancers would not be possible, and therefore vital prognostic information would be unavailable and access to adjuvant therapy limited.
At the time of writing, there are no published cases reporting simultaneous use of 2 sentinel lymph node biopsy techniques. However, 1 other case report has been published documenting the overall management of a patient with concurrent primary melanoma of the back and breast cancer. The authors highlight a lack of data on the incidence of synchronous primary malignancies and the paucity of evidence in the management of the lymph node basins. Interestingly, there are reports in the literature that demonstrate an increased risk of developing a second primary malignancy after developing malignant melanoma;9 however, no current guidance exists for managing such cases.6 Not only is this case helpful in the future development of guidance for similar cases, but it will also help guide surgeons when a similar, challenging dual-malignancy diagnosis is made and mapping of the sentinel lymph nodes are required.
Our case successfully demonstrates the simultaneous use of 2 SLNB mapping techniques, without interference or complication. We advocate for simultaneous mapping of the lymph node basins in such cases to reduce the possibility of false negatives secondary to changes in the lymphatic drainage following surgery,10 reduce the overall number of procedures required by the patient, and provide timely histological results to enable further adjuvant therapy.
Acknowledgments
Authors: Murtaza Kadhum, MBBS, BSc, MSc, MRCS, MBA1; Parinita Swarnkar, BSc, MBBS1; Marianne Dillon, MBBS, FRCS (General Surgery)2; Sarah Hemington-Gorse, MB, BCh, FRCS (Plastic Surgery)1
Affiliations: 1Burns and Plastic Surgery, Morriston Hospital, Swansea, Wales, United Kingdom; 2 Breast Surgery, Morriston Hospital, Swansea, Wales, United Kingdom
Correspondence: Murtaza Kadhum, MBBS, BSc, MSc, MRCS, MBA; Murtaza.kadhum2@nhs.net
Ethics: The patient provided informed consent for this case report.
Disclosures: The authors disclose no financial or other conflicts of interest.
References
1. Cancer Research UK. Cancer incidence for common cancers. Accessed July 8, 2023. https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#collapseZero.
2. Mirzaei N, Katsarelias D, Zaar P, et al. Sentinel lymph node localization and staging with a low-dose of superparamagnetic iron oxide (SPIO) enhanced MRI and magnetometer in patients with cutaneous melanoma of the extremity - The MAGMEN feasibility study. Eur J Surg Oncol. 2022;48(2):326-332. doi:10.1016/J.EJSO.2021.12.467
3. Yang J, Xu L, Liu P, et al. Accuracy of sentinel lymph node biopsy in breast cancer: pitfalls in the application of single tracers. Cancer Manag Res. 2020;12:3045. doi:10.2147/CMAR.S244806
4. National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and management. Published June 14, 2023. Accessed July 5, 2023. https://www.nice.org.uk/guidance/ng101/chapter/Recommendations.
5. National Institute for Health and Care Excellence. Magtrace and Sentimag system for locating sentinel lymph nodes for breast cancer. Published 2022. Accessed July 5, 2023. https://www.nice.org.uk/guidance/mtg72.
6. Fosko NK, Davis CH, Koshenkov VP, Kowzun MJ. Simultaneous primary invasive breast carcinoma and ipsilateral cutaneous melanoma of the back: surgical approach and considerations, a case report. Int J Surg Case Rep. 2021;84:106155. doi:10.1016/J.IJSCR.2021.106155
7. National Institute for Health and Care Excellence. Melanoma: assessment and management. Published 2022. Accessed July 5, 2023. https://www.nice.org.uk/guidance/ng14/chapter/Recommendations-for-research.
8. Temple-Oberle C, Nicholas C, Rojas-Garcia P. Current controversies in melanoma treatment. Plast Reconstr Surg. 2023;151(3):495E-505E. doi:10.1097/PRS.0000000000009936
9. Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol. 2010;146(3):265-272. doi:10.1001/ARCHDERMATOL.2010.2
10. Johnson C, Intenzo C, Mastrangelo MJ, Feeney K, Berger AC. Altered drainage patterns in patients with melanoma and previous axillary dissection. J Dermatol. 2013;40(7):564-566. doi:10.1111/1346-8138.12143