Regenerative Peripheral Nerve Interfaces for the Management of Symptomatic Non-extremity Neuropathic Pain and Neuromas
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Introduction. Regenerative peripheral nerve interfaces (RPNI) can be used to mitigate neuropathic pain resulting from nerve injury or neuroma formation following trauma, surgery, or amputation. Most of the current literature discusses the utility of RPNI for the treatment of neuropathic pain in the upper and lower extremities; however, RPNI can also improve neuropathic pain in non-extremity regions. Our objective was to characterize and describe patient cases of non-extremity RPNIs.
Methods. We retrospectively reviewed medical records of patients treated with RPNIs for non-extremity neuropathic pain by the senior author at a single institution between February 2020 and October 2023.
Cases. Seven patients were treated with RPNI in non-extremity regions. For 1 patient, RPNI was performed prophylactically following discovery of injured peripheral nerves during a surgery and the patient did not report any neuropathic pain in the related regions afterwards. Six patients presented with nerve pain in multiple regions, including the scalp, face, trunk, and groin, that began either after a trauma or previous surgery. The nerve pain of 5 patients completely resolved after the creation of an RPNI.
Discussion. The creation of an RPNI is relatively straightforward and can relieve or prevent peripheral nerve pain caused by injured nerves. While RPNIs have mainly been used for the treatment or prevention of neuromas in extremities, this case series demonstrates efficacy in non-extremity areas as well. Surgeons can, therefore, consider RPNI for patients who have neuropathic pain due to suspected nerve injury that has been refractory to other treatments.
Introduction
Injury to the peripheral nerves from trauma or surgery can lead to the formation of neuromas and chronic neuropathic pain.1 Surgically induced neuropathic pain is estimated to occur in 10% to 50% of patients after common operations.2,3 Patients may complain of intermittent or constant pain (generally in the path of a known nerve) including paresthesia, allodynia, and hyperalgesia. The pain can be refractory to medical treatment and is associated with lower self-reported quality of life.4,5
Regenerative peripheral nerve interface (RPNI) is a novel surgical technique that improves neuropathic pain. The RPNI is created by implanting the divided end of a peripheral nerve into a free muscle graft.6 Most research on RPNI focuses on effectiveness in treating nerve pain in the extremities, but it can also be beneficial for treating nerve injuries in other areas of the body, such as the face or abdomen.7-15 In this case series, we discuss how RPNI can improve neuropathic pain in non-extremity regions.
Seven patients treated with RPNIs for non-extremity neuropathic pain at a single institution between February 2020 and October 2023 by the senior author were included (Table). Their average age was 42.8 ± 15.2 years. Indications for RPNI included neuropathic pain (n = 6) and prophylaxis, where a transected peripheral nerve from a prior operative intervention was identified (n = 1). Causes of neuropathic pain ranged from trauma or past surgeries in the region.

In all cases, if pain improved after a nerve block at the point of maximal tenderness, it was deemed that the pain was likely neuropathic, and RPNI was offered. Prior to surgery, the path of pain was marked on the skin (Figure 1A). During surgery, the nerve was identified along the path of pain and divided (Figure 1B). A freed segment of local or distant muscle was then wrapped around the distal end of the nerve.

Figure 1. (A) The marking of the path of pain on the skin. (B) Isolating the nerve found in the marked path of pain.
Cases
RPNI in the head and face
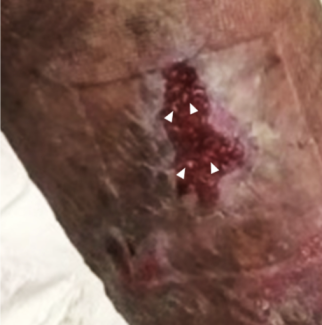
Case 1. A 42-year-old patient presented with pain in the right upper lip and nasal sill region following a motor vehicle accident 18 months prior. It was deemed she likely developed a neuroma of a terminal branch of the maxillary nerve. During surgery, the nerve at the point of maximal tenderness was placed into a segment of orbicularis muscle (Figure 2). After surgery, she reported excellent relief of pain.

Figure 2. RPNI with orbicularis muscle for neuroma in the upper lip region.
Case 2. A 47-year-old patient presented with pain in the superior aspect of his right ear that radiated to the side of his head whenever he wore glasses or a mask. He had experienced this pain for 2 years following ear reconstruction after a house fire. A selective denervation of the right auriculotemporal nerve branch was successfully performed with RPNI using a segment of the temporalis muscle.
RPNI in the trunk
Case 3. A 58-year-old patient presented with left abdominal wall pain present since a vascular bypass (left epigastric artery to left dorsal artery of the penis) surgery 18 years prior. During surgery, the identified nerve was placed into a segment of rectus muscle. After surgery, he experienced scarring and a deep aching pain, which he could not distinguish from his preoperative pain. He failed to return for follow up after 5 weeks, so it remains unknown if the underlying nerve pain improved.
Case 4. A 46-year-old patient presented with sharp pain in the left lower quadrant for 4 years. The pain was mostly alleviated after an inguinal mass excision, where a lipoma was found to be prolapsing into the internal ring 3 years prior to presentation. The etiology of the persistent neuropathic pain remained unclear. During surgery, a nerve piercing the rectus sheath medially was identified, transected, and placed into a free segment of rectus muscle. Afterwards, the patient’s neuropathic pain was completely gone.
Case 5. An 11-year-old patient presented with 1 year of burning and shooting left-sided chest pain that began after surgical excision of a left rib for fibrous dysplasia. During surgery, the intercostal nerve was divided distally and placed into a segment of freed pectoralis muscle. Her preoperative pain was greatly improved.
RPNI in the hip and groin
Case 6. A 41-year-old patient with necrotizing fasciitis underwent left hip disarticulation. Two weeks later, during a subsequent debridement, scarred nerve ends were found. The sciatic nerve and a cluster of middle cluneal nerves were trimmed back to healthy nerve and wrapped with a free segment of local muscle. The patient had no complaints of neuropathic pain in the regions supplied by the sciatic or middle cluneal nerves, although he did later develop phantom limb pain.
Case 7. A 52-year-old patient presented with 1.5 years of shooting left inguinal pain that began after an open left inguinal hernia repair with mesh. During RPNI surgery, the genitofemoral nerve was identified near the point of maximal tenderness, and the divided end was placed into a free segment of internal oblique muscle. He developed some localized aching, though his nerve pain completely resolved.
Discussion
The creation of an RPNI is a straightforward and quick surgical technique that helps relieve neuroma pain. Unlike other existing surgical methods such as neuroma excision alone, nerve capping, nerve implantation into a vein, or fibrin patch applications with local pain catheters, RPNI is a physiological technique. RPNI involves the use of a free muscle graft to provide targets for nerve regeneration, preventing the formation of painful nerve sprouting.16,17
The indications for RPNI are variable. RPNI was originally developed to provide control signals for prosthetic devices.16-18 An anatomical and technical guide discusses RPNI techniques in the extremities and briefly addresses its use for thoracic and abdominal neuromas; other case reports discuss its use in headache and breast surgery.6,19,20 Most reported cases involve the treatment of neuromas in the extremities or amputated limbs.7-15
We discuss the treatment of 7 patients with RPNI in a non-extremity area. Of the 6 patients who presented with neuropathic pain, 5 patients demonstrated diminished neuropathic pain after surgery. Patient 3 had an aching pain after the surgery. He did not seek medical attention regarding continued pain after his last follow-up 5 weeks after surgery. It is therefore possible, but difficult to verify, that his neuropathic pain improved over time. Unlike the other patients, Patient 6 received RPNI as a prophylactic measure, demonstrating another utility of RPNI. This indication is consistent with the literature that supports the use of RPNI to prevent neuroma formation in patients with amputated limbs12-14and nerve pain in sensate anterolateral thigh flap donor sites.21
Five patients presented with neuropathic pain following a surgical procedure. The rate of chronic postsurgical pain varies greatly depending on the type of surgery, affecting 3% to 85% of patients.22 About 10% of surgical patients develop severe pain that significantly impairs daily functioning.22 The pain may be somatic, visceral, or neuropathic in nature, with nerves being trapped or injured during surgery. There are no universally accepted guidelines for the management of postsurgical pain, but options include watchful waiting, physical therapy, medications, nerve blocks, and additional surgery.23
Patients in our case series often trialed different pain medications and sought help from other nonsurgical and surgical providers without finding relief until undergoing RPNI. When neuropathic pain is suspected and other treatments have failed, RPNI may be a suitable option. Furthermore, altered postoperative abnormalities are rarely a concern to patients as the zone of skin sensation is already altered and painful from the initial trauma, and it could be possible that local nerves are sprouting and filling any cortical gaps in the time between injury and RPNI creation. It is the senior author’s standard practice to share that there could be worsened local numbness; however, patients typically do not mind if their neuropathic pain is alleviated.
Overall, we propose the use of RPNIs for the treatment of symptomatic neuromas and neuropathic pain in non-extremity regions. As opposed to other surgical techniques like targeted nerve implantation or targeted muscle reinnervation, RPNIs can be constructed without specialized microsurgical or peripheral nerve training. Furthermore, even if the recipient site does not have suitable tissue, muscle grafts can be harvested from a distant donor site with little morbidity.8,18
This study’s limitations include small sample size, variability in neuroma location, and inconsistent follow-up. Additional studies with larger patient populations and longer, more consistent follow-up periods are warranted to better elucidate the therapeutic effect of RPNIs in non-extremity regions. A prospective study for patients with non-extremity neuropathic pain treated with RPNI is imperative to explore pain onset, frequency, severity, medication use, and quality of life measures. A prospective study can also explore potential complications, including numbness.
Conclusions
This case series suggests that RPNI is a safe and efficacious technique to attenuate neuropathic pain thought to be caused by damaged nerves in non-extremity regions. Providers should consider RPNI when patients have neuropathic pain, potentially following trauma, surgery, or amputation, that is refractory to conservative measures. Further studies with larger sample sizes should be conducted to evaluate the effectiveness of RPNI in this population.
Acknowledgements
Authors: Navya Baranwal, BA1; Jesse E. Menville, BA1; Elijah M. Persad-Paisley, BA1; Nikhil Sobti, MD2; Loree K. Kalliainen, MD, MA2
Affiliations: 1The Warren Alpert Medical School of Brown University, Providence, Rhode Island; 2Division of Plastic Surgery, The Warren Alpert Medical School of Brown University, Providence, Rhode Island
Correspondence: Navya Baranwal; navya_baranwal@brown.edu
Ethics: The study was exempt from review by Rhode Island Hospital Lifespan’s Institutional Review Board due to the limited number of patients included in the series.
Disclosures: The authors report no financial conflict of interest concerning the materials or methods used or the findings specified in this paper.
References
1. Yang H, Dong Y, Wang Z, et al. Traumatic neuromas of peripheral nerves: Diagnosis, management and future perspectives. Front Neurol. 2022;13:1039529. doi:10.3389/fneur.2022.1039529
2. Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. Mar 2013;257(3):403-412. doi:10.1097/SLA.0b013e3182701a7b
3. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. May 13 2006;367(9522):1618-1625. doi:10.1016/S0140-6736(06)68700-X
4. O'Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27(2):95-112. doi:10.2165/00019053-200927020-00002
5. Parsons B, Schaefer C, Mann R, et al. Economic and humanistic burden of post-trauma and post-surgical neuropathic pain among adults in the United States. J Pain Res. 2013;6:459-469. doi:10.2147/JPR.S44939
6. Leach GA, Dean RA, Kumar NG, et al. Regenerative peripheral nerve interface surgery: anatomic and technical guide. Plast Reconstr Surg Glob Open. Jul 2023;11(7):e5127. doi:10.1097/GOX.0000000000005127
7. Hooper RC, Cederna PS, Brown DL, et al. Regenerative peripheral nerve interfaces for the management of symptomatic hand and digital neuromas. Plast Reconstr Surg Glob Open. Jun 2020;8(6):e2792. doi:10.1097/GOX.0000000000002792
8. Woo SL, Kung TA, Brown DL, Leonard JA, Kelly BM, Cederna PS. Regenerative peripheral nerve interfaces for the treatment of postamputation Neuroma Pain: A Pilot Study. Plast Reconstr Surg Glob Open. Dec 2016;4(12):e1038. doi:10.1097/GOX.0000000000001038
9. Sayegh A, Jaloux C, Witters M, Mayoly A, Kachouh N. Update on upper limb neuroma management. J Craniofac Surg. May 01 2023;34(3):1140-1143. doi:10.1097/SCS.0000000000009164
10. Richards JT, Baird MD, Tintle SM, Souza JM, Renninger CH, Potter BK. Peripheral nerve management in extremity amputations. Orthop Clin North Am. Apr 2022;53(2):155-166. doi:10.1016/j.ocl.2022.01.002
11. Santosa KB, Oliver JD, Cederna PS, Kung TA. Regenerative peripheral nerve interfaces for prevention and management of neuromas. Clin Plast Surg. Apr 2020;47(2):311-321. doi:10.1016/j.cps.2020.01.004
12. Kubiak CA, Kemp SWP, Cederna PS, Kung TA. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast Reconstr Surg. Sep 2019;144(3):421e-430e. doi:10.1097/PRS.0000000000005922
13. Mauch JT, Kao DS, Friedly JL, Liu Y. Targeted muscle reinnervation and regenerative peripheral nerve interfaces for pain prophylaxis and treatment: A systematic review. PMR. Mar 25 2023;doi:10.1002/pmrj.12972
14. Lin Z, Yu P, Chen Z, Li G. Regenerative peripheral nerve interface reduces the incidence of neuroma in the lower limbs after amputation: a retrospective study based on ultrasound. J Orthop Surg Res. Aug 24 2023;18(1):619. doi:10.1186/s13018-023-04116-6
15. Pejkova S, Nikolovska B, Srbov B, et al. Prophylactic regenerative peripheral nerve interfaces in elective lower limb amputations. Pril (Makedon Akad Nauk Umet Odd Med Nauki). Apr 22 2022;43(1):41-48. doi:10.2478/prilozi-2022-0004
16. Kung TA, Bueno RA, Alkhalefah GK, Langhals NB, Urbanchek MG, Cederna PS. Innovations in prosthetic interfaces for the upper extremity. Plast Reconstr Surg. Dec 2013;132(6):1515-1523. doi:10.1097/PRS.0b013e3182a97e5f
17. Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. Jun 2014;133(6):1380-1394. doi:10.1097/PRS.0000000000000168
18. Woo SL, Urbanchek MG, Cederna PS, Langhals NB. Revisiting nonvascularized partial muscle grafts: a novel use for prosthetic control. Plast Reconstr Surg. Aug 2014;134(2):344e-346e. doi:10.1097/PRS.0000000000000317
19. Gfrerer L, Wong FK, Hickle K, Eberlin KR, Valerio IL, Austen WG. RPNI, TMR, and reset neurectomy/relocation nerve grafting after nerve transection in headache surgery. Plast Reconstr Surg Glob Open. Mar 2022;10(3):e4201. doi:10.1097/GOX.0000000000004201
20. Hart SE, Agarwal S, Hamill JB, Brown DL. Effective treatment of chronic mastectomy pain with intercostal sensory neurectomy. Plast Reconstr Surg. May 01 2022;149(5):876e-880e. doi:10.1097/PRS.0000000000008975
21. Isbester KA, Ferrin P, Krakauer KN, Peters BR. Primary regenerative peripheral nerve interfaces using devascularized vastus lateralis muscle in sensate anterolateral thigh flap donor sites. Plast Reconstr Surg Glob Open. Sep 2023;11(9):e5241. doi:10.1097/GOX.0000000000005241
22. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. Apr 13 2019;393(10180):1537-1546. doi:10.1016/S0140-6736(19)30352-6
23. Thapa P, Euasobhon P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. Jul 2018;31(3):155-173. doi:10.3344/kjp.2018.31.3.155