Postsurgical Pyoderma Gangrenosum After Penile Inversion Vaginoplasty: Case With Review of Diagnostic and Management Strategies
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background. Postsurgical pyoderma gangrenosum (PSPG) is a highly uncommon and unpredictable wound healing complication. Rapid progression of ulcers at incisions can cause unfettered dehiscence. Most commonly, PSPG involves breast procedures; however, in this work, we detail a case of a patient who developed PSPG 10 days postoperatively after penile inversion vaginoplasty.
Methods. The patient in this case underwent a penile inversion vaginoplasty with orchiectomy in the standard fashion. She had no risk factors for PSPG. Following an uncomplicated hospital stay, the patient developed difficulty with pain control and increasing serous drainage on the 10th postoperative day. On readmission, the patient was found to have developed large, mildly purulent ulcers throughout the perineal wound edges. On exam under anesthesia, the neovaginal canal was found to be patent and intact. The dehisced portions of the incisions were left open and redressed with occlusive bismuth-petrolatum dressing. Dermatology was promptly consulted with suspicion for PSPG. The patient was started on an 18-day prednisone taper with cyclosporine, along with doxycycline and ciprofloxacin.
Results. After 5 days of immunosuppressive treatment, the ulcers visibly converted to healthy granulation tissue and were no longer actively purulent. Following another washout, the dehisced wound edges were reapproximated. At follow-up, the patient had no evidence of PSPG recurrence and continued dilating on schedule. Our patient recovered from PSPG without further complications and a satisfactory aesthetic result.
Conclusions. This unique case highlights the importance of prompt dermatological consultation, immunosuppression, and avoidance of further pathergy in the setting of suspicion for PSPG.
Introduction
Penile inversion vaginoplasty (PIV) is the most commonly performed technique in genital feminization surgery. While it does not generally require free flaps or utilization of surgical microscopes as in phalloplasty, PIV still requires extensive tissue rearrangement, specifically the creation of a neovaginal canal and dissection of periurethral tissue. These added complexities make urological and rectal complications common in PIV but comparable to genitourinary reconstruction procedures. Some studies report a minor complication rate as high as 70%,1,2 and approximately 20% to 25% undergo revision procedures, most commonly follow-up labioplasties and urethroplasties.3 Minor wound disruption and dehiscence of higher tension points in the reconstruction are considered the most common complications within 30 days postoperatively.1-2
Wound dehiscence is generally driven by a combination of mechanical and physiological factors, including local ischemia, infection, and wound-disrupting comorbidities. Postsurgical pyoderma gangrenosum (PSPG), however, is an uncommon and potentially devastating cause of postoperative dehiscence and complete loss of reconstruction. Pyoderma gangrenosum (PG) generally presents as papules that quickly evolve into purulent ulcers with rolled, violaceous borders driven by neutrophil overactivity. These ulcers can rapidly erode tissue and dehisce incisions. Often first mistaken for infection, debridement and the resulting pathergy paradoxically worsen PSPG. Many patients that develop PSPG have some form of underlying inflammatory disease, particularly ulcerative colitis. PSPG can create an unpredictable and difficult challenge in the domain of reconstructive surgery and has been most reported in literature after breast procedures, which account for approximately 25% of reported cases of PSPG.4 Most reported cases of PSPG in breast procedures have occurred after breast reduction, followed by microsurgical breast reconstruction.4 In this work, however, we report a case of PSPG after PIV, a particularly rare and previously unreported complication that led to significant perineal dehiscence 10 days after initial surgery.
Methods
Our patient is a transgender woman in her 20s with no relevant past medical or surgical history besides 2 years of progesterone/estradiol hormone therapy (HRT). Her body mass index at the time of surgery was 27.7 kg/m2. She specifically lacked any relevant risk factors for PG, namely no prior history of PG, known inflammatory bowel diseases, or hematological/rheumatological disorders. She underwent a single-stage penile inversion vaginoplasty in 2022 with indicated orchiectomy, penectomy, clitoroplasty, and labiaplasty.
Procedure
The vaginoplasty procedure followed the typical course of a PIV without any intraoperative or immediate perioperative complications. The location for the neovaginal introitus was marked out from the perineal body with an inferiorly based skin flap, and a section of skin from the perineum posterior to the scrotum was removed down to the deep dermis/subcutaneous tissue junction for later use for coverage in the neovaginal canal and to gain access for the orchiectomy. Following orchiectomy, the bulbospongiosus muscle was dissected completely from the spongy urethra. The pelvic floor muscles were cut laterally and longitudinally at the tendinous junction using Bovie cautery to increase the size of the neovaginal introitus. To create space for the neovaginal canal, the Denonvilliers fascia was bluntly dissected to create an approximately 8-inch depth separation between the prostatic capsule and the rectum. The neoclitoris was constructed from the dorsal glans and the corpora were dissected from the penis. The remaining Buck fascia was folded in an accordion fashion and collectively sutured with the neoclitoris to the pubic bone. The skin of the penis and the extra section of perineal skin were sutured together to completely cover a silicone dilator, which was then inserted into the neovaginal cavity to create the neovaginal canal. Both the neoclitoris and the shortened urethra were brought through an incision in the anterior skin of the public area and sewed together and secured to the surrounding tissue. Two 15-French round drains were placed on either side of the groin incision. All remaining skin incisions were finally closed with 3-0 monofilament synthetic absorbable deep dermal and 4-0 vertical mattress sutures. The neovaginal canal was packed and the incisions were covered with bismuth-petrolatum fine mesh occlusive dressing, and sterile abdominal pads with cotton padding were taped down over the surgical site in a bolster fashion, further secured with mesh underwear.
Postoperative course
Postoperatively, the patient had no signs of major or minor complications. Notably, on postoperative day 3, she briefly developed a low-grade fever and serosanguinous saturation of her rear dressing with minimal drainage into her 2 wound drains, which was stable for the remainder of her stay. The foam taped dressings and the intravaginal packing were removed on postoperative day 5, and she was discharged with oral antibiotics and oral pain medications. She was instructed to clean the surgical site with regular soap and water and use the silicone dilator with water-based lubricant as part of standard post-vaginoplasty dilation therapy.
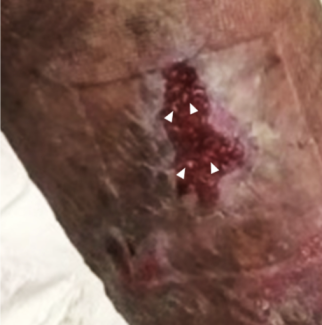
On postoperative day 10 and prior to outpatient follow-up, however, the patient developed intermittent chills, increasing difficulty with perineal pain control, and substantial serous fluid drain output and dressing saturation. On return to the hospital, she was found to have mild leukocytosis with a neutrophil predominance but was afebrile and hemodynamically stable. Subsequent physical exam revealed that the neovaginal canal was patent and intact. There was near total skin dehiscence of the perineal wounds, particularly in the lateral suture lines of the new labia majora. Larger lesions connected between the lateral suture lines and the neovaginal canal, so maceration from the vaginal packing, dressing, and/or drains appeared unlikely. Additionally, areas of the skin that contacted dressing tape appeared to be free of any irritation. On subsequent exploration under anesthesia, the bilateral inguinal fold wound edges displayed mildly purulent scalloping with rolled edges and sharp erythematous borders (Figure 1) that quickly raised suspicion for PSPG. Wound cultures were taken from the lesions in the operating room. Debridement was not deemed necessary. The dehisced sections of the wound were left open, and the patient was redressed with bismuth-petrolatum fine mesh occlusive dressing, abdominal pads, and mesh underwear. The dermatology service was consulted and concurred with PSPG assessment. In the setting of high clinical suspicion, we did not perform a biopsy of the lesions in the interest of avoiding further pathergy. The patient was started on cyclosporine with an 18-day prednisone taper. As there was a low index of suspicion for an active infection/necrotizing fasciitis (NF) in the setting of immunosuppression, doxycycline and ciprofloxacin were started.

Figure 1. Examination under anesthesia revealed considerable dehiscence of the incisions surrounding the new labia majora and neoclitoris. The new vaginal canal was found to be patent and intact.
Wound cultures later grew few multi-drug resistant extended-spectrum beta-lactamase (ESBL) E. coli resistant to ciprofloxacin. In the absence of findings consistent with surgical site infection, this was deemed most likely due to contamination or colonization of the wound given the culture was performed at bedside and taken from the perineum. Urine and blood cultures were ultimately negative. As part of the dermatology service workup, herpes simplex virus 2 (HSV-2) IgG was positive with no recent evidence of lesions or prodrome per patient history. Valacyclovir was started preemptively.
Results
With treatment, the patient's leukocytosis, wounds, and pain control improved. There was no evidence of expansion of the PSPG ulcers. After 5 days of immunosuppressive treatment, the wounds were no longer purulent and had visibly converted to healthy granulation tissue (Figure 2). The patient returned to the OR and underwent washout with delayed primary closure of the open wounds with resorbable suture. The previously ulcerated lateral aspects of the perineal wound were reapproximated by tucking in the newer granulation tissue. The patient was deemed safe for discharge 9 days after readmission. At follow-up, the patient had some minor skin breakdown at the groin creases and was briefly started on topical tacrolimus (0.1% topical ointment). These areas quickly improved and were deemed instead to be flexural eczema. Approximately 20 days after discharge, there was no evidence of continued ulceration or recurrence of PSPG (Figure 3). At 3-month postoperative follow-up, the patient continued dilating on schedule without difficulty and without any evidence of PSPG recurrence.

Figure 2. Perineal wounds prior to washout and reapproximation on day 5 of immunosuppressive treatment. Ulcers had halted growth and were minimally purulent with conversion to healthy granulation tissue.

Figure 3. Well-healed and closed perineal wounds at first follow-up, approximately 3 weeks after reapproximation with resorbable suture.
Discussion
PG can be a destructive and highly unpredictable postoperative cause of wound dehiscence. This is especially true in the setting of reconstructive plastic surgery where procedures like PIV involve extensive and delicate tissue rearrangement.
PG is classified as a reactive, noninfectious neutrophilic dermatosis. It is a particularly rare diagnosis with a roughly estimated incidence of under 10 cases per million5 and typically affects middle-aged adults. Biological males and females are thought to be affected roughly equally, but some studies have found a significant female predominance.6 PG may have a genetic component, as cases have been observed to cluster among family members.7,8 Due to its rarity, understanding and evolution of the general management of PSPG has been heavily driven by anecdotal information and systematic reviews of case series rather than observational or interventional studies. Yet, PSPG has an apparent association specifically with breast procedures,4 making it a particularly important complication to identify for the reconstructive surgeon. However, PSPG is often mistaken for wound infection, treated with debridement, and consequently worsened by the resulting pathergy.
While the exact cause of PG is unknown and its occurrence is usually spontaneous, many patients have underlying systemic inflammatory disease. Most often this comes in the form of inflammatory bowel disease, classically ulcerative colitis. Seronegative spondyloarthropathies are also commonly implicated, and PSPG may have an association with hematologic malignancies.9,12 Our patient had no significant medical or rheumatological history besides 2 years of progesterone/estradiol HRT. There is currently no evidence to suggest any association of PG with HRT use. As this is the first reported case of PSPG in the context of gender affirming surgery, it is difficult to assess whether patients, including those with autoimmune conditions, are at relatively increased risk for developing PSPG after PIV. While this patient did have a positive HSV-2 IgG, we believe this was very unlikely to be the cause of the skin ulceration, which was very inconsistent with HSV. The patient did not report any prior history of herpetic lesions and there were no prodromal symptoms prior to this case.
The rapid onset of uncontrolled pain, neutrophilia, and formation of ulcers in the perioperative period in this case initially led to some concern for infection. Early identification/differentiation and management of PSPG versus wound infection/necrotising fasciitis (NF) is understandably critical due to the stark difference in management but similarity in gross presentation and initial severity. Clinically, PSPG typically presents 4 days to several weeks into the postoperative period.4 Grossly, it presents with painful, purulent ulcers that appear at or near the wound border and can quickly cause dehiscence of the surgical site. It may also present with foul-smelling discharge, further confounding the diagnosis. As was seen in this case, the ulcer classically has a rolled violaceous and dusky red border. Unfortunately, no tests or histology can definitively confirm PG versus NF—it is a clinical diagnosis of exclusion.10 Sterile neutrophilia on histopathology can help secure the diagnosis,10 but this must be weighed against introducing further pathergy with a biopsy. NF typically improves with rapid source control; however, further pathergy can greatly worsen the progression of PSPG. PSPG is generally considered aseptic, but superimposed infection or wound colonization is common. In this case, the ESBLs that grew in the wound culture were likely from wound colonization/contamination rather than active infection. Nonetheless, NF lacks the characteristic rolled violet edge, and PG generally only develops significant tissue necrosis in more severe, progressed cases. In NF, superficial fascia also characteristically separates easily to blunt dissection. NF and PG are similarly very uncommon. NF has an incidence of up to approximately 50 per million11 and PG has a roughly estimated incidence of up to 10 per million per year in the United States.5 This further underscores the need for a high degree of clinical suspicion for PSPG.
When there is concern for PSPG, urgent dermatological consultation and immunosuppression rather than surgical intervention favors good prognosis. In our case, medical therapy was a combination of prednisone taper and cyclosporine. In the literature, prednisone and prednisolone are most documented.12,13 Topical steroid therapies have also been used with effect. Oral cyclosporine is becoming more frequently used in PG as a monotherapy or steroid adjunct.13,14 Tacrolimus, another macrolide lactone immunosuppressant, has shown efficacy as a topical ointment in patients with PG refractory to steroid therapy.13 As such, topical tacrolimus was used in this case when there was some concern for recurrence after prednisone taper. Other steroid-sparing immunosuppressive agents have been reported to successfully halt disease progression. Refractory PG has demonstrated response to many biologic immunomodulators, especially anti-tumor necrosis factor monoclonal antibodies such as infliximab.14 These newer therapies could be a valuable adjunct in highly refractory PSPG and could reduce or eliminate the need for substantial future reconstruction/revision.
Specialized wound management in PSPG is critical to prevent secondary infection, reduce inflammation, and promote ulcer re-epithelialization. In this case, we initially treated our patient with broad spectrum oral antibiotics as we were immunosuppressing a patient with large, exposed wounds in the perineum and wound cultures positive for ESBLs. Perineal wounds carry a high risk of colonization. However, there were no overt signs of infection (ie, cellulitis) and the wounds were not particularly purulent or malodorous considering their size. In the literature, routine use of prophylactic systemic antibiotics in PG cases where there are not many signs of active soft tissue infection is generally not recommended.15 Topical antibiotics and antiseptics are also generally not recommended for routine use in colonized PG wounds.15 Antimicrobial dressings are helpful in managing colonized PG wounds. While bismuth-petrolatum fine mesh occlusive dressing was used in the case of smaller minimally exudative ulcers, occlusive dressings are generally not recommended for large, rapidly progressive ulcers with a strong exudative component.15,16 In such cases, allogeneic/autologous skin grafting combined with negative pressure wound therapy have shown early favorable results when combined with immunosuppression.15,16
Due to the proximity of the vaginoplasty wound edges to the urethra, rectum, and the intraperitoneal space, dehiscence from PSPG could lead to complications such as large fistulas into the rectum, pelvis, and urological structures. Fortunately, in this case, PSPG was quickly identified as the likely diagnosis. With prompt medical treatment and wound care followed by nonsurgical reapproximation of the wound edges, there was no substantial loss of tissue in the vaginoplasty either within or outside the operative site. The patient is doing well at long-term follow-up and continuing to dilate without difficulty.
Acknowledgments
Authors: Michael M. Talanker, BS; Jessica R. Nye, BS; David T. Mitchell, MD; Daniel J. Freet, MD, FACS
Affiliation: Division of Plastic Surgery, Department of Surgery, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, Texas
Correspondence: Michael Talanker, BS; michael.m.talanker@uth.tmc.edu
Ethics: This case is reported with the explicit, informed consent of the patients.
Disclosures: The authors disclose no relevant financial or nonfinancial interests.
References
1. Hontscharuk R, Alba B, Hamidian Jahromi A, Schechter L. Penile inversion vaginoplasty outcomes: Complications and satisfaction. Andrology. 2021;9(6):1732-1743. doi:10.1111/andr.13030
2. Ferrando CA. Adverse events associated with gender affirming vaginoplasty surgery. Am J Obstet Gynecol. 2020;223(2):267.e1-267.e6. doi:10.1016/j.ajog.2020.05.033
3. Boas SR, Ascha M, Morrison SD, et al. Outcomes and predictors of revision labiaplasty and clitoroplasty after gender-affirming genital surgery. Plast Reconstr Surg. 2019;144(6):1451-1461. doi:10.1097/PRS.0000000000006282
4. Zuo KJ, Fung E, Tredget EE, Lin AN. A systematic review of post-surgical pyoderma gangrenosum: identification of risk factors and proposed management strategy. J Plast Reconstr Aesthet Surg. 2015;68(3):295-303. doi:10.1016/j.bjps.2014.12.036
5. Monari P, Moro R, Motolese A, et al. Epidemiology of pyoderma gangrenosum: Results from an Italian prospective multicentre study. Int Wound J. 2018;15(6):875-879. doi:10.1111/iwj.12939
6. Xu A, Balgobind A, Strunk A, Garg A, Alloo A. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sex-adjusted population analysis. J Am Acad Dermatol. 2020;83(2):425-429. doi:10.1016/j.jaad.2019.08.001
7. Alberts JH, Sams HH, Miller JL, King LE Jr. Familial ulcerative pyoderma gangrenosum: a report of 2 kindred. Cutis. 2002;69(6):427-430.
8. DeFilippis EM, Feldman SR, Huang WW. The genetics of pyoderma gangrenosum and implications for treatment: a systematic review. Br J Dermatol. 2015;172(6):1487-1497. doi:10.1111/bjd.13493
9. Conrad C, Trüeb RM. Pyoderma gangrenosum. J Dtsch Dermatol Ges. 2005;3(5):334-342. doi:10.1111/j.1610-0387.2005.05022.x
10. Bisarya K, Azzopardi S, Lye G, Drew PJ. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! A case report and literature review. Eplasty. 2011;11:e24.
11. Arif N, Yousfi S, Vinnard C. Deaths from necrotizing fasciitis in the United States, 2003-2013. Epidemiol Infect. 2016;144(6):1338-1344. doi:10.1017/S0950268815002745
12. Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. BMJ. 2006;333(7560):181-184. doi:10.1136/bmj.333.7560.181
13. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53(2):273-283. doi:10.1016/j.jaad.2004.10.006
14. Maronese CA, Pimentel MA, Li MM, Genovese G, Ortega-Loayza AG, Marzano AZ. Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol. 2022 Sept;23(5):615-634.
15. Croitoru D, Naderi-Azad S, Sachdeva M, Piguet V, Alavi A. A wound care specialist's approach to pyoderma gangrenosum. Adv Wound Care (New Rochelle). 2020;9(12):686-694. doi:10.1089/wound.2020.1168
16. Pichler M, Thuile T, Gatscher B, et al. Systematic review of surgical treatment of pyoderma gangrenosum with negative pressure wound therapy or skin grafting. J Eur Acad Dermatol Venereol. 2017;31(2):e61-e67. doi:10.1111/jdv.13727