Limb Salvage in Extensive Necrotizing Soft Tissue Infection with Adjuvant Hyperbaric Oxygen Therapy
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
A necrotizing soft tissue infection (NSTI) can be life-threatening if not treated promptly, posing a high risk of limb amputation. Here, we report a case of an NSTI extending from the buttocks and perineum down to the left lower limb. The case involved a 48-year-old male patient who presented with fever, altered consciousness, and limb swelling. Computed tomography showed the infection had spread to the perifascial, intermuscular, and intramuscular regions, making it difficult to save the patient's life and limb. Despite prompt surgery and antibiotic treatment, multidrug-resistant bacteria presented difficult wound management challenges. Hyperbaric oxygen therapy (HBO) was initiated, which resulted in dramatic wound improvement and successful skin grafting. Due to limb preservation, the patient was able to recover his preadmission activities of daily living and successfully reintegrate into society. Standard treatments for NSTI include early surgical treatment, antibiotics, and intensive support. The adjunctive use of HBO therapy may have contributed to the successful outcome in this case.
Introduction
Necrotizing soft tissue infection (NSTI) remains a highly lethal disease associated with high rates of limb amputations. It is crucial to initiate appropriate treatments for this disease without delay, including surgery, antibiotic administration, and systemic management. Here, we report a case of a severe NSTI extending from the perineum to the lower limbs. The extent of the infection posed a difficult challenge to saving the patient's life and limb. However, with the addition of hyperbaric oxygen therapy (HBO) to conventional treatment, limb preservation was achieved, and the patient was successfully reintegrated into his routine activities.
Case Presentation
A 48-year-old man was brought to our hospital via emergency transport due to fever, altered consciousness, and swelling of the left lower limb of 2 days' duration. He had a history of lower body paresis caused by spina bifida and was previously treated at another hospital for an ischial pressure ulcer. Five years ago, he was treated for Fournier gangrene (FG). His activities of daily living (ADL) consisted of independently transferring himself to a wheelchair, standing, and walking short distances with an orthosis, and crawling indoors. Upon physical examination, the patient was noted to be in a state of pre-shock with a Glasgow Coma Scale score of 8 (E1V3M4), body temperature of 37.1ºC, blood pressure of 112/68 mm Hg, heart rate of 112 beats per minute, and an oxygen saturation of 95% (room air). His blood test results were as follows: C-reactive protein, 46.54 mg/dL; white blood cells, 17,160/μL; hemoglobin, 13.5 g/dL; hematocrit, 41.1%; sodium, 136 mmol/L; potassium, 3.2 mmol/L; creatinine, 2.32 mg/dL; glucose, 193 mg/dL; and bicarbonate, 19.2 mmol/L. Computed tomography revealed extensive gas accumulation in the perifascial, intermuscular, and intramuscular regions of the left buttock, perineum, thigh, and lower leg (Figure 1). Therefore, the diagnosis of NSTI was made.

Figure 1. Computed tomography scans showing extensive gas images in the perifascial, intermuscular, and intramuscular regions, extending down from the left buttock (arrow) to the perineum, left thigh, and left lower leg.
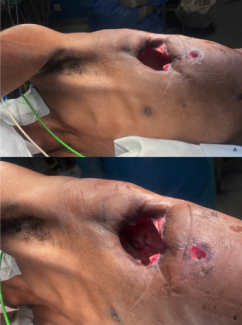
On the first day of hospitalization, the patient underwent emergency surgical intervention. Skin and fascial incisions were urgently made, and the wound areas were extensively exposed and debrided. A large amount of sludge-like pus and necrotic tissue was observed under the fascia (Figure 2). Some portions of the muscles were also necrotic. Significant bleeding was encountered during the procedure; therefore, only the infected sites were opened for drainage. Blood loss amounted to 1970 mL, and 2 units of red blood cells were transfused during the operation.

Figure 2. Patient photographs on the first day of hospitalization. Left photo shows obvious swelling of the left lower limb. Right photo demonstrates the lateral thigh incision, showing large amounts of sludge-like pus under the fascia.
After surgery, the patient was placed under general care in the intensive care unit, and additional debridement and bilateral orchiectomy were performed on the third day of hospitalization. The patient soon regained respiratory and hemodynamic stability and was extubated on the 10th day.
Methicillin-sensitive Staphylococcus aureus, Escherichia coli, and Clostridium ramosum were detected in the wound bacterial cultures collected during the initial surgery. Treatment was consequently switched from broad-spectrum antibiotics (meropenem, vancomycin, and clindamycin [CLDM]) to those tailored for sensitivity (cefazolin and CLDM). Around the 20th hospital day, the exudate drastically increased and was of a vivid green hue, viscous, and foul-smelling; therefore, microbial substitution was suspected. Multidrug-resistant Pseudomonas aeruginosa and Corynebacterium spp were detected from the exudate, and the antibiotics were changed. However, little improvement was observed. At this point, there was no necrotic tissue in the wound, and the muscles were exposed (Figure 3). Therefore, the detection of resistant bacteria alone did not lead to additional debridement. Instead, hyperbaric oxygen therapy (HBO), a minimally invasive treatment, was started on the 37th hospital day. HBO sessions were performed for 60 minutes at a pressure of 2.0 atmosphere absolute (ATA). After just 1 session, the foul-smelling exudate significantly decreased. As the color, tone, and edema of the granulation tissue improved (Figure 3), HBO therapy was discontinued after 10 sessions.

Figure 3. Patient limb photographs taken on the 19th hospital day, the 39th hospital day (after 2 sessions of hypobaric oxygen [HBO] therapy), and the 47th hospital day (after 9 sessions of HBO), respectively, from left to right. Proliferation of granulation tissue and improvement of color and tone are observed.
Split-thickness skin grafting was performed on the patient's lower left limb on the 54th hospital day. The skin graft successfully adhered to the wound, leading to a significant reduction in the wound area (Figure 4). The patient gradually regained his ability to perform ADLs at his previous level, including the ability to propel his wheelchair by himself and crawl indoors. He was consequently discharged on his 101st hospital day.
Figure 4. Patient limb photograph demonstrating the success of the skin grafting and the healed wound.
Discussion
An NSTI is a serious infection that can rapidly progress and can be life-threatening if not treated promptly. The speed at which necrosis progresses is thought to be around 2 to 3 cm per hour.1 Despite advances in NSTI diagnosis and treatment, the mortality rate remains high, with approximately 30% to 50% of patients deteriorating into septic shock2 for which the mortality rate is estimated to be around 30% or higher.3-5 Delays in initiating appropriate treatment are associated with increased mortality.6 NSTI of perineal lesions, as seen in this case, is classified as FG, with a reported mortality rate of 20% to 50%.7 NSTI also carries a high risk of limb amputation, which is required in approximately 50% of patients.3
There are severity indices for FG, such as the Fournier's Gangrene Severity Index (FGSI)8 and the Uludag Fournier's Gangrene Severity Index (UFGSI).9 FGSI, a scoring system developed in 1995 based on 30 cases of FG, is used to assess the severity and prognosis of patients. The UFGSI is a scoring system that adds the extent of gangrene and patient age to the FGSI based on 80 cases studied in 2007 and is considered to reflect the prognosis more accurately (Table 2).9 A score >9 is associated with a mortality rate of 94%, whereas a score of ≤9 is associated with a survival rate of 81%. In this case, the UFGSI score was 15, which is associated with very poor outcomes.

The standard treatments for NSTI include early and thorough surgery, appropriate antibiotics, and systemic management. As a proposed adjunctive treatment for NSTI, the use of HBO has potentially beneficial effects in patients. HBO is a treatment method that involves the inhalation of 100% oxygen under pressure >1 ATA to increase tissue oxygen levels.10 The general effects of HBO include the reduction of neutrophil endothelial adhesion in acute ischemia and reperfusion injury; correction of tissue hypoxia; improvement of tissue microcirculation11; direct bactericidal effects of oxygen5,10,12,13; promotion of leukocytic sterilization3,4,12; potentiation of antibiotics3,5,10,12,13; reduction of edema10; and enhancement of wound healing through the promotion of angiogenesis,4,10,12,14 fibroblast proliferation,3,4,10,12,14 collagen production,10 and epithelialization.10 HBO also has immunomodulatory effects that decrease pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α and increase the anti-inflammatory cytokine IL-10.4,13 In biofilm infections, local oxygen deprivation promotes the uptake and downregulation of antibiotic targets. The effect of HBO on the reoxygenation of biofilm-associated infections has been demonstrated in experimental models, suggesting that HBO may be effective against biofilms.2
However, clear evidence of the benefit of HBO for NSTI has not yet been fully established. In the 10th European Consensus Conference on Hyperbaric Medicine, HBO for the treatment of NSTI was strongly recommended, whereas the level of evidence supporting it was low.15 Some reports described that HBO reduced mortality and amputation rates, while others reported that there was no significant difference (Table 1). NSTI is a life-threatening disease, and randomized controlled trials are difficult to conduct for ethical reasons. However, the use of HBO in the treatment of NSTI remains controversial. Further accumulation of cases and research are expected in the future.

In the present case, despite a protracted course of wound healing, the limb was saved with the judicious use of HBO as adjunctive therapy. While the appropriate administration of antibiotics and prompt surgery are the basis of treatment for NSTI, HBO may have contributed to the improvement of infection and promotion of wound healing in this case. Considering that NSTI still incur high rates of limb amputation and mortality, effective adjunctive therapies must be integrated into conventional treatments to improve treatment outcomes, and HBO is conceivable as one such option.
Acknowledgments
Authors: Kaori Yauchi, MD1; Natsuki Shikata, MD1; Yoshie Shibaoka, MD1
Affiliations: Department of Plastic Surgery, Kohnan Medical Center, Hyogo, Japan
Correspondence: Kaori Yauchi, MD; kaoriyauchi@gmail.com
Ethics: This is an observational study. The Research Ethics Committee of our institution has confirmed that no ethical approval is required. The human subject has given informed consent for the use of photographs.
Disclosures: The authors have no relevant financial or nonfinancial interests to disclose.
References
1. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):e119-125. doi:10.1002/bjs.9371
2. Hedetoft M, Jensen PØ, Moser C, Vinkel J, Hyldegaard O. Hyperbaric oxygen treatment impacts oxidative stress markers in patients with necrotizing soft-tissue infection. J Investig Med Off Publ Am Fed Clin Res. 2021;69(7):1330-1338. doi:10.1136/jim-2021-001837
3. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg Chic Ill 1960. 2004;139(12):1339-1345. doi:10.1001/archsurg.139.12.1339
4. Huang C, Zhong Y, Yue C, He B, Li Y, Li J. The effect of hyperbaric oxygen therapy on the clinical outcomes of necrotizing soft tissue infections: a systematic review and meta-analysis. World J Emerg Surg WJES. 2023;18(1):23. doi:10.1186/s13017-023-00490-y
5. Faunø Thrane J, Ovesen T. Scarce evidence of efficacy of hyperbaric oxygen therapy in necrotizing soft tissue infection: a systematic review. Infect Dis Lond Engl. 2019;51(7):485-492. doi:10.1080/23744235.2019.1597983
6. Martinschek A, Evers B, Lampl L, Gerngroß H, Schmidt R, Sparwasser C. Prognostic aspects, survival rate, and predisposing risk factors in patients with Fournier's gangrene and necrotizing soft tissue infections: evaluation of clinical outcome of 55 patients. Urol Int. 2012;89(2):173-179. doi:10.1159/000339161
7. Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg WJES. 2018;13:58. doi:10.1186/s13017-018-0219-9
8. Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier's gangrene. J Urol. 1995;154(1):89-92.
9. Yilmazlar T, Ozturk E, Ozguc H, Ercan I, Vuruskan H, Oktay B. Fournier's gangrene: an analysis of 80 patients and a novel scoring system. Tech Coloproctology. 2010;14(3):217-223. doi:10.1007/s10151-010-0592-1
10. Aydın F, Kaya A, Savran A, İncesu M, Karakuzu C, Öztürk AM. Diabetic hand infections and hyperbaric oxygen therapy. Acta Orthop Traumatol Turc. 2014;48(6):649-654. doi:10.3944/AOTT.2014.3225
11. Hedetoft M, Moser C, Jensen PØ, Vinkel J, Hyldegaard O. Soluble ICAM-1 is modulated by hyperbaric oxygen treatment and correlates with disease severity and mortality in patients with necrotizing soft-tissue infection. J Appl Physiol Bethesda Md 1985. 2021;130(3):729-736. doi:10.1152/japplphysiol.00844.2020
12. Steiner T, Seiffart A, Schumann J, Bucher M. Hyperbaric oxygen therapy in necrotizing soft tissue infections: a retrospective study. Adv Exp Med Biol. 2018;1072:263-267. doi:10.1007/978-3-319-91287-5_42
13. Hedetoft M, Garred P, Madsen MB, Hyldegaard O. Hyperbaric oxygen treatment is associated with a decrease in cytokine levels in patients with necrotizing soft-tissue infection. Physiol Rep. 2021;9(6):e14757. doi:10.14814/phy2.14757
14. Lee SS, Sun JH, Chang LY, Ueng SW, Shih CH. Limb-threatening necrotizing alternariosis salvaged by adjunctive hyperbaric oxygen therapy. Scand J Infect Dis. 1998;30(2):194-196. doi:10.1080/003655498750003645
15. Mathieu D, Marroni A, Kot J. Tenth European Consensus Conference on Hyperbaric Medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb Med. 2017;47(1):24-32. doi:10.28920/dhm47.1.24-32
16. Gibson A, Davis FM. Hyperbaric oxygen therapy in the management of Clostridium perfringens infections. N Z Med J. 1986;99(808):617-620.
17. Riseman JA, Zamboni WA, Curtis A, Graham DR, Konrad HR, Ross DS. Hyperbaric oxygen therapy for necrotizing fasciitis reduces mortality and the need for debridements. Surgery. 1990;108(5):847-850.
18. Brown DR, Davis NL, Lepawsky M, Cunningham J, Kortbeek J. A multicenter review of the treatment of major truncal necrotizing infections with and without hyperbaric oxygen therapy. Am J Surg. 1994;167(5):485-489. doi:10.1016/0002-9610(94)90240-2
19. Shupak A, Oren S, Goldenberg I, Barzilai A, Moskuna R, Bursztein S. Necrotizing fasciitis: An indication for hyperbaric oxygenation therapy? Surgery. 1995;118(5):873-878. doi:10.1016/S0039-6060(05)80278-8
20. Hollabaugh RSJ, Dmochowski RR, Hickerson WL, Cox CE. Fournier's gangrene: therapeutic impact of hyperbaric oxygen. Plast Reconstr Surg. 1998;101(1):94.
21. Dahm P, Roland FH, Vaslef SN, et al. Outcome analysis in patients with primary necrotizing fasciitis of the male genitalia. Urology. 2000;56(1):31-35; discussion 35-36. doi:10.1016/s0090-4295(00)00604-x
22. Mindrup SR, Kealey GP, Fallon B. Hyperbaric oxygen for the treatment of Fournier's gangrene. J Urol. 2005;173(6):1975-1977. doi:10.1097/01.ju.0000158129.56571.05
23. George ME, Rueth NM, Skarda DE, Chipman JG, Quickel RR, Beilman GJ. Hyperbaric oxygen does not improve outcome in patients with necrotizing soft tissue infection. Surg Infect. 2009;10(1):21-28. doi:10.1089/sur.2007.085
24. Hassan Z, Mullins RF, Friedman BC, et al. Treating necrotizing fasciitis with or without hyperbaric oxygen therapy. Undersea Hyperb Med J Undersea Hyperb Med Soc Inc. 2010;37(2):115-123.
25. Soh CR, Pietrobon R, Freiberger JJ, et al. Hyperbaric oxygen therapy in necrotising soft tissue infections: a study of patients in the United States Nationwide Inpatient Sample. Intensive Care Med. 2012;38(7):1143-1151. doi:10.1007/s00134-012-2558-4
26. Massey PR, Sakran JV, Mills AM, et al. Hyperbaric oxygen therapy in necrotizing soft tissue infections. J Surg Res. 2012;177(1):146-151. doi:10.1016/j.jss.2012.03.016
27. Shaw JJ, Psoinos C, Emhoff TA, Shah SA, Santry HP. Not just full of hot air: hyperbaric oxygen therapy increases survival in cases of necrotizing soft tissue infections. Surg Infect. 2014;15(3):328-335. doi:10.1089/sur.2012.135
28. Li C, Zhou X, Liu LF, Qi F, Chen JB, Zu XB. Hyperbaric oxygen therapy as an adjuvant therapy for comprehensive treatment of Fournier's gangrene. Urol Int. 2015;94(4):453-458. doi:10.1159/000366137
29. Devaney B, Frawley G, Frawley L, Pilcher DV. Necrotising soft tissue infections: the effect of hyperbaric oxygen on mortality. Anaesth Intensive Care. 2015;43(6):685-692. doi:10.1177/0310057X1504300604
30. Hung MC, Chou CL, Cheng LC, et al. The role of hyperbaric oxygen therapy in treating extensive Fournier's gangrene. Urol Sci. 2016;27(3):148-153. doi:10.1016/j.urols.2015.06.294
31. Anheuser P, Mühlstädt S, Kranz J, Schneidewind L, Steffens J, Fornara P. Significance of hyperbaric oxygenation in the treatment of Fournier's gangrene: a comparative study. Urol Int. 2018;101(4):467-471. doi:10.1159/000493898
32. Feres O, Feitosa MR, Ribeiro da Rocha JJ, et al. Hyperbaric oxygen therapy decreases mortality due to Fournier's gangrene: a retrospective comparative study. Med Gas Res. 2021;11(1):18-23. doi:10.4103/2045-9912.310055
33. Tutino R, Colli F, Rizzo G, et al. Which role for hyperbaric oxygen therapy in the treatment of Fournier's gangrene? A retrospective study. Front Surg. 2022;9:850378. doi:10.3389/fsurg.2022.850378
34. Mladenov A, Diehl K, Müller O, von Heymann C, Kopp S, Peitsch WK. Outcome of necrotizing fasciitis and Fournier's gangrene with and without hyperbaric oxygen therapy: a retrospective analysis over 10 years. World J Emerg Surg WJES. 2022;17(1):43. doi:10.1186/s13017-022-00448-6