Effectiveness of Using Autologous Saphenous Vein as Arteriovenous Graft for Patients With Chronic Wounds Undergoing Hemodialysis
© 2024 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Although the first choice of vascular access for hemodialysis is still the creation of an arteriovenous fistula, the increasing number of vascular access creations in Japan, as well as the inability of percutaneous transluminal angioplasty to effectively restore patency in stenotic and obstructed native vascular accesses, has resulted in an increase in the rate of arteriovenous grafting for hemodialysis. However, for patients with infected wounds, using a prosthetic graft is generally contraindicated because of the risk of the spread of infection. In such cases, options tend to be limited to less frequently used or novel methods of vascular hemodialysis access, such as basilic vein transposition and autologous vein transplantation. Herein, we report 3 successful cases of arteriovenous grafting using an autologous saphenous vein. Careful preoperative evaluation of the vascular anatomy is necessary to effectively determine the best option for vascular access in such patients.
Introduction
In Japan, the number of vascular access creations is increasing every year because of the increase in diabetic nephropathy, advanced age at which dialysis is initiated, and lengthening of the dialysis period.1 Stenosis and obstruction of vascular accesses occur as a result of frequent punctures. For such cases, percutaneous transluminal angioplasty (PTA) can be performed but does not maintain patency. Thus, the use of arteriovenous grafts is increasing.2 However, for patients with infected wounds, placement of a prosthetic arteriovenous graft is not recommended because of the possibility of the spread of infection to the graft.3 In such cases, options tend to be limited to less frequently used or novel methods of vascular hemodialysis access, such as basilic vein transposition and autologous vein transplantation4.Herein, we report 3 cases using the autologous saphenous vein as an arteriovenous graft for hemodialysis, with good results, and include the specific surgical technique performed.
Case Presentations
Case 1
A 44-year-old male patient X-13 years, due to acute renal failure from trauma, underwent arteriovenous graft insertion using a prosthetic graft(polytetrafluoroethylene PTFE) and continuous dialysis was started. At X-1 years, the patient developed chronic infection with calciphylaxis. He developed graft infection, cellulitis, and pseudoaneurysm formation due to frequent punctures. To control the infection, the graft was removed and an arteriovenous fistula was created on the contralateral wrist. However, the venous system was thin, measuring only 1.5 mm in preoperative ultrasound, and the fistula was occluded. Due to the chronic infection, prosthetic grafts could not be used. One month later (X years), an arteriovenous graft was created via great saphenous vein transplantation. End-to-side anastomosis was performed between the distal transplanted vein and radial artery, while an end-to-end anastomosis was performed between the proximal transplanted vein and basilic vein. Dialysis was performed without any problems for 5 years after the operation (X + 5 years).
Case 2
A 71-year-old male patient. One month prior, the patient underwent toe amputation after percutaneous transluminal angioplasty for chronic limb-threatening ischemia on the foot. Wound infection was persistent, renal function deteriorated, and hemodialysis was initiated. Ultrasound revealed that an arteriovenous fistula could not be created because the cephalic veins on both the right and left sides were obstructed. Thus, the patient underwent arteriovenous graft placement via great saphenous vein transplantation. End-to-side anastomosis was performed between the distal transplanted vein and radial artery, while an end-to-end anastomosis was performed between the proximal transplanted vein and ulnar cutaneous vein. Although the transplanted vein developed mild stenosis, hemodialysis was performed successfully without PTA for 2 years after the operation.
Case 3
A 69-year-old male patient. One year prior, the patient underwent amputation of the right 3rd to 5th toes due to chronic limb-threatening ischemia on the right lower extremity. Due to lack of blood flow, the wound became an intractable ulcer and chronic infection developed. Renal failure worsened during wound treatment and hemodialysis was initiated. Preoperative ultrasound showed that the cutaneous veins in the forearm were thin (median vein of 2.0 mm), making arteriovenous fistula creation difficult. A month later, an arteriovenous graft using the autologous saphenous vein was inserted. End-to-side anastomosis of the greater saphenous vein and radial artery and end-to-end anastomosis of the greater saphenous vein and brachial ulnar vein were performed. However, physiological stenosis of the radial artery at its origin from the brachial artery resulted in weak blood flow, and the graft was occluded. Thus, arteriovenous graft insertion was again performed, still using autologous vein. End-to-side anastomosis of the greater saphenous vein and ulnar artery and end-to-end anastomosis of the greater saphenous vein and ulnar vein of the brachial ulnar vein were performed. After several dialysis sessions, it was again occluded. PTA with stenting was performed 6 months after the operation, resulting in successful dialysis.
Methods
The method of arteriovenous grafting via autologous vein transplantation was as follows. The procedure was started by marking the radial artery and the large cutaneous vein (Figure 1). Approximately 30 cm of the great saphenous vein was harvested (Figure 2) from the lower leg using interrupted incisions to prevent skin necrosis (Figure 3). Careful ligation of the branches was performed to prevent stenosis. The harvested vein was prepared by adventitial dissection of stenotic areas and dilation of the vein using saline solution. A subcutaneous tunnel was then created to pass the great saphenous vein in the forearm, ensuring that the vein was not twisted (Figure 4).

Figure 1. Preoperative markings. Marking of the radial artery and the large cutaneous vein (basilic vein, Case 3) is performed.
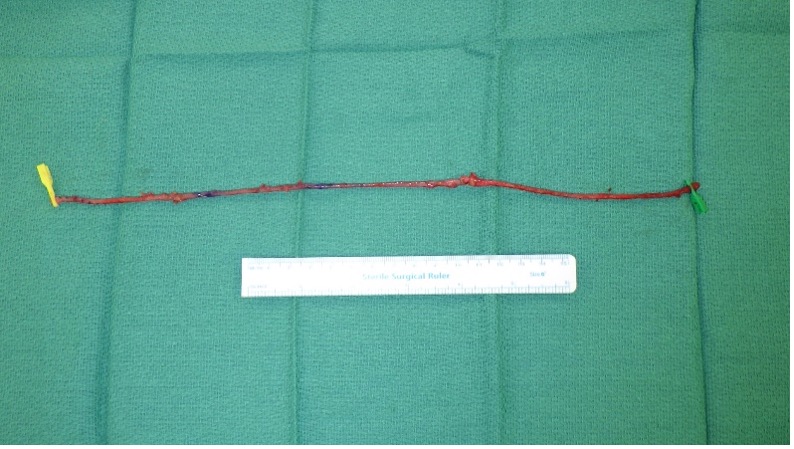
Figure 2. The harvested great saphenous vein. A length of 30 cm is taken in order to create a loop. Branches are carefully ligated to avoid stenosis. Before suturing, saline solution is injected to dilate the great saphenous vein. Areas likely to be stenosed are dissected from the adventitia.

Figure 3. Left lower leg from which the great saphenous vein was harvested. Interrupted incisions that leave some skin prevent necrosis of the wound margin.

Figure 4. Right forearm at the end of the anastomosis. A subcutaneous tunnel is created to pass the great saphenous vein, carefully ensuring that the harvested saphenous vein is not twisted.

Figure 5. Ultrasound image 5 years after bypass surgery in Case 3. The anastomosis between the radial artery and the great saphenous vein is patent, and there is no stenosis in the grafted vein.
Discussion
In these cases, all patients had severe chronic limb-threatening ischemia and chronic wounds. Thus, we created arteriovenous grafts using an autologous vein that is less susceptible to infection. Although postoperative PTA was performed in 1 case, all patients were able to successfully undergo hemodialysis.
The priority for initial vascular access creation at the time of dialysis initiation remains an arteriovenous fistula. In more than 95% of cases, the above techniques can be used. If none of these are possible, an arteriovenous fistula using the ulnar artery may be considered, but it should be avoided as it often results in shunt problems and is difficult to puncture.5
The radial and ulnar arteries are identified as branches from the brachial artery and are evaluated on the forearm. It is important to assess the wall properties, thickness, presence of calcification, and arterial blood flow rate using vascular ultrasound.
An arterial diameter of 2.0 mm or greater is recommended for creation of an arteriovenous fistula between the distal radial artery and cephalic vein of the non-dominant forearm;6-8 1.6 to 1.9 mm is sufficient for creation, but less than 1.5 mm is associated with lower success rates.6-9 Meanwhile, the diameter of the vein should be at least 1.6 to 2.5 mm. Arteriovenous fistulas with a vein diameter of 2.0 mm or greater have a patency rate of 76%, whereas those with a vein diameter of less than 2.0 mm have a significantly lower patency rate of only 16%.9 Therefore, preoperative ultrasonography is important. Cases 1 and 3 had very thin veins measuring 2.0 mm in diameter when the veins were dilated by hemodiafiltration. Case 2 had obstruction of both forearm veins, making arteriovenous fistula creation in the forearm difficult.
In cases where the veins are too small to create an autologous arteriovenous fistula, options for vascular access include arteriovenous graft insertion using prosthetic grafts, superficialization of the brachial artery, and long-term indwelling catheters. The technique should be selected after preoperative evaluation of the patient's general condition and peripheral circulation.
Indications for arteriovenous graft placement are patients with cardiac function capable of withstanding excessive blood flow, difficulty in puncture either due vessel stenosis or thick subcutaneous tissues, and damaged autologous arteriovenous fistula.10 Complications of arteriovenous graft insertion include increased cardiac load, infection, and pseudoaneurysm of the vessel. Approximately 90% of infections of artificial vessels are caused by Methicillin-resistant Staphylococcus aureus and are intractable. Pseudoaneurysms are likewise intractable and often must be removed.11 Moreover, arteriovenous grafts and long-term indwelling catheters are not recommended in cases of infected wounds.3
There have been many reports of basilic vein transposition (BVT) at the brachial level,12-14 but the procedure involves an anastomosis with the more central brachial artery and may cause dialysis access-associated steal syndrome. We opted to perform vascular access creation using the radial artery, which is a peripheral artery. If the transplanted great saphenous vein were to become occluded, it would still be possible to perform BVT at the brachial level.
The advantages of using an autologous vein for arteriovenous grafting include: (1) the absence of foreign material, allowing its use in patients with chronic wounds, and (2) faster hemostasis and less risk for pseudoaneurysm formation compared to prosthetic grafts. Meanwhile, the disadvantages include: (1) longer operative time; (2) delayed wound healing in patients with chronic limb-threatening ischemia (CLTI) because the great saphenous vein is harvested; and (3) the possibility of thrombus formation due to the pliability and bending of the autologous vein. It has been reported that the use of arteriovenous graft insertion using an autologous vein versus the use of polytetrafluoroethylene has higher primary (93% vs 88%, respectively, in the 12th month; 82% vs 56%, respectively, in 24th month) and secondary (96% vs 96%, respectively, in 12th month; 93% vs 84%, respectively, in 24th month) patency rates and has fewer complications at 2 years postoperatively.15
Conclusions
Three cases of arteriovenous graft insertion using an autologous vein graft performed at our institution were presented. We believe that arteriovenous grafting using autologous veins, together with BVT, can be performed for cases in which a prosthetic graft cannot be implanted due to chronic infected wounds. Careful evaluation of the presence of stenosis, vessel caliber, blood flow, and wall calcification before surgery is desirable to determine the appropriate site for arteriovenous anastomosis.
Acknowledgments
Authors: Masahiro Kuwabara, MD, PhD; Hiroto Hosoyamada, MD; Kana Tokuno, MD; Takahiro Hirayama, MD; Eri Ichijo, MD; Naoto Yamamoto, MD, PhD
Affiliation: Department of Plastic and Reconstructive Surgery, Saitama Medical Center, Jichi Medical University
Correspondence: Masahiro Kuwabara, MD, PhD; kuwaichinai@gmail.com
We would like to thank Editage (www.editage.com) for English language editing.
Ethics: IRB approval was not required. The patients provided informed consent for the procedure.
Disclosures: The authors disclose no relevant financial or nonfinancial interests.
References
1. Sato T, Sakurai H, Okubo K, Kusuta R, Onogi T, Tsuboi M. Current state of dialysis treatment and vascular access management in Japan. J Vasc Access. 2019;20(1_suppl):10-14. doi:10.1177/1129729819838183
2. Tsuchida K, Nagai K, Minakuchi J, Kawashima S. Vascular access for long-term hemodialysis/hemodiafiltration patients in Japan. Contrib Nephrol. 2015;185:132-137. doi:10.1159/000380977.
3. Izumi Y. Countermeasures against infection in critical limb ischemia treatments. Jpn J Vasc Surg. 2018;27:129-132
4. Harris JP, & May J. Autogenous saphenous vein grafts as vascular access for hemodialysis. In: Andreucci VE, ed. Vascular and Peritoneal Access for Dialysis. Topics in Renal Medicine, vol. 8. Springer; 1989:45-54.
5. Liu W, Lagaac R, Pettigrew GJ, Callaghan CJ. Outcomes after ulnar-basilic arteriovenous fistula formation. Ann Vasc Surg. 2013;27(2):232-237. doi:0.1016/j.avsg.2012.04.014
6. Malovrh M. Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis. 2002;39(6):1218-1225. doi:10.1053/ajkd.2002.33394
7. Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg. 1996;12(2):207-213. doi:10.1016/s1078-5884(96)80108-0
8. Silva MB Jr, Hobson RW 2nd, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27(2):302-308. doi:10.1016/s0741-5214(98)70360-x
9. Mendes RR, Farber MA, Marston WA, Dinwiddie LC, Keagy BA, Burnham SJ. Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J Vasc Surg. 2002;36(3):460-463. doi:10.1067/mva.2002.126544
10. Hiranaka T. Tapered and straight grafts for hemodialysis access: a prospective, randomized comparison study. In: Henry ML, ed. Vascular Access for Hemodialysis VII. W. L. Gore & Associates; 2001:219-225.
11. Hisata Y, Inoue T, Tasaki Y, Odate T, Yamada T. Management of arteriovenous graft infection. Ann Vasc Dis. 2022;15(4):282-288. doi:10.3400/avd.oa.22-00058
12. Koudounas G, Giannopoulos S, Houser A, Karkos C, Volteas P, Virvilis D. Basilic vein tunnel transposition versus elevation transposition for brachiobasilic arteriovenous fistula creation: a systematic review and meta-analysis. J Vasc Access. 2024;11297298241226993. doi:10.1177/11297298241226993
13. Masood B, Batool Zaidi SA, Alam S, Mir S. Single stage versus two stage basilic vein transposition for hemodialysis access: a retrospective observational study. J Vasc Access. 2023;11297298231210952. doi:10.1177/11297298231210952
14. Abdo EM, Abouelgreed TA, Elshinawy WE, et al. Use of basilic vein in arteriovenous fistulas construction for hemodialysis access. Is it a good option alternative to prosthetic arteriovenous grafts? Arch Ital Urol Androl. 2023;95(3):11455. doi:10.4081/aiua.2023.11455
15. Uzun A, Diken AI, Yalçinkaya A, et al. Long-term patency of autogenous saphenous veins vs. PTFE interposition graft for prosthetic hemodialysis access. Anadolu Kardiyol Derg. 2014:14(6):542-546. doi:10.5152/akd.2014.4910
















