Alkali Burns of the Skin
© 2023 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Questions
-
What are the common causes of cutaneous alkali burns?
-
How does aqueous sodium hydroxide interact with the skin?
-
What is the immediate management of alkali burns?
-
What surgical treatment is warranted for the patient depicted in Figure 1?
Case Description
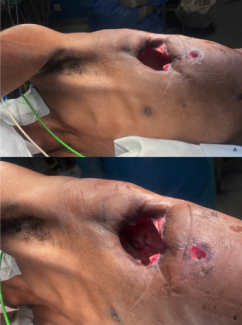
During the preparation of a chemical experiment, a 21-year-old laboratory technician accidentally splashed sodium hydroxide solution onto his face. Despite immediately washing the affected area with tap water for 5 minutes, he experienced a burning sensation on his forehead and noticed it was red. Two hours later, the patient sought medical attention at the emergency department, where he was noted to have a grey dry eschar over the forehead that extended onto his upper eyelids and nose (Figure 1). Analgesia was prescribed, and the wound and eyes were thoroughly irrigated with saline. The patient later underwent tangential excision of the burn eschar and the wound was resurfaced with a split-thickness skin autograft.

Q1. What are the common causes of cutaneous alkali burns?
Alkalis are soluble hydroxides of the alkali metals (eg, sodium, potassium, and lithium), the soluble hydroxides of the alkaline earth metals (eg, calcium, strontium, and barium), and compounds that produce hydroxides in solution, such as ammonia.
Sodium hydroxide and aqueous ammonia are used in industrial and household cleaning products due to their effectiveness in breaking down organic material, such as grease. They are components of oven and car cleansing materials, paint thinners, and drain cleaners.1
Exposure to anhydrous ammonia, which is used in the production of fertilizers, refrigerators, air-conditioning systems, explosives, petroleum, plastics, pesticides, and synthetic fibers, is linked to deep cutaneous burns, severe ophthalmic and pulmonary injuries, and agricultural accidents.2 Anhydrous ammonia is also a crucial component in the production of illegal methamphetamine.3

Workers in the construction sector and do-it-yourself enthusiasts can develop painless deep burns after kneeling in wet cement or concrete since they contain calcium oxide, which dissolves in water to form calcium hydroxide (Figure 2).4 Alkalis have also been implicated in chemical assaults.5
The ignition of lithium batteries in electronic cigarettes can cause alkali burns, as lithium compounds, such as lithium cobalt oxides or lithium manganese oxides, react with water to form lithium hydroxide and hydrogen gas in an exothermic reaction.6
Q2. How does aqueous sodium hydroxide interact with the skin?
Alkalis can injure the skin by 3 mechanisms.1 Firstly, they can saponify fat, a process involving the hydrolysis of ester bonds in the lipids of the stratum corneum and the phospholipids of the cell membrane. This process generates carboxylate salts of fatty acids (soaps) and glycerol, which reduces the skin’s permeability barrier and enhances the penetration of alkali (Figure 3). Secondly, alkaline hydrolysis of proteins disrupts peptide bonds resulting in the formation of alkaline proteinates (Figure 4). This leads to the destruction of the 3-dimensional molecular structure of proteins (denaturation) causing liquefactive necrosis. Lastly, alkalis possess hygroscopic properties that can dehydrate tissues which, coupled with exothermic reactions, result in cell death.


Q3. What is the immediate management of alkali burns?
Prompt removal of alkali from the skin is crucial, as the presence of excess hydroxide ions can cause the burn to progress. All clothing that is in contact with the affected area must be removed and the burns irrigated with copious amounts of saline or water. Powdered calcium oxide (lime) should be brushed off the wound before water is applied. Application before irrigation of Diphoterine, an amphoteric chelating molecule that binds hydroxide ions, has been shown to improve outcomes.7
The rationale behind irrigation is to eliminate the alkali, reduce the pH, and suppress the hygroscopic reaction. Fluid resuscitation should be initiated for burns greater than 20% total body surface area, with early consideration of endotracheal intubation for burns of the face and pharynx. Irrigation should continue until the pH is near neutral, which can take more than 2 hours. Delayed Irrigation beyond 10 minutes is ineffective in limiting the extension of tissue destruction.8 Reports dating back to 1927 have advised against neutralizing alkaline burns with acid due to the risk of deepening the injury through an exothermic acid-base reaction.9 However, experiments in a rat model demonstrated that neutralization of sodium hydroxide with 5% acetic acid (household vinegar) reduced the extent of tissue injury.10 A complete eye examination is mandatory for facial injuries. Ophthalmic consultation should be sought as necessary, and the irrigation of the ocular surface continued until the pH of the conjunctival sac is below 8.5. A sterile balanced salt solution is preferable to tap water and can be delivered through IV tubing or a Morgan lens and delivery system.11
Q4. What surgical treatment is warranted for the patient depicted in Figure 1?
The burn on the patient’s forehead (Figure 1) was deep and required early excision and skin grafting. A guarded knife was used to excise the eschar until diffuse punctate bleeding was seen, which was controlled by the application of epinephrine soaks. The scalp was chosen as the donor site since this was likely to result in the best texture and color match. Preoperatively, the head was shaved and the frontal hairline was marked to avoid inadvertently harvesting skin from the forehead.12 The scalp was infiltrated in the subgaleal plane with 1:500,000 epinephrine and a split-thickness skin graft harvested with a Zimmer dermatome set at 0.0018 inches. The handpiece was moved from ear to ear over the scalp keeping the blade at a 45° angle to the skin while an assistant supported the head. Greater amounts of skin may be harvested more conveniently with the use of the Mayfield neurosurgical headrest.13 The graft was then gently rinsed in normal saline to remove any hairs, secured to the forehead as a single esthetic unit using fibrin glue,14 and protected with Xeroform (Figure 5A). The donor scalp was also dressed with Xeroform, which was allowed to separate spontaneously over the next 2 weeks as the scalp regenerated and hair regrew (Figure 5B). Long-term follow-up revealed a satisfactory cosmetic and functional outcome (Figure 5C).

Acknowledgments
Affiliation: Professor of Plastic and Reconstructive Surgery, Johns Hopkins University School of Medicine, Baltimore, MD (Ret.)
Correspondence: Stephen M Milner, MBBS, BDS, DSc (Hon), FRCSE, FACS; stephenmilner123@gmail.com
Ethics: Informed consent was provided for use of images.
Disclosures: The author discloses no relevant conflict of interest or financial disclosures for this manuscript.
References
1. Greenwood JE, Tan JL, Ming JC, Abell AD. Alkalis and skin. J Burn Care Res. 2016;37(2):135-141. doi:10.1097/BCR.0000000000000222
2. White CE, Park MS, Renz EM, et al. Burn center treatment of patients with severe anhydrous ammonia injury: Case reports and literature review. J Burn Care Res. 2007;28(6):922-928. doi:10.1097/BCR.0b013e318159a44e
3. Bloom GR, Suhail F, Hopkins-Price P, Sood A. Acute anhydrous ammonia injury from accidents during illicit methamphetamine production. Burns. 2008;34(5):713-718. doi:10.1016/j.burns.2007.08.015
4. Rao PP, Menezes RG. Cement burns. Burns. 2009;35(2):308-309. doi:10.1016/j.burns.2008.05.031
5. Young RC, Ho WS, Ying SY, Burd A. Chemical assaults in Hong Kong: a 10-year review. Burns. 2002;28(7):651-653. doi:10.1016/s0305-4179(02)00096-7
6. Nicoll KJ, Rose AM, Khan MA, Quaba O, Lowrie AG. Thigh burns from exploding e-cigarette lithium ion batteries: First case series. Burns. 2016;42(4):e42-e46. doi:10.1016/j.burns.2016.03.027
7. Donoghue AM. Diphoterine for alkali chemical splashes to the skin at alumina refineries. Int J Dermatol. 2010;49(8):894-900. doi:10.1111/j.1365-4632.2009.04397.x
8. Yano K, Hata Y, Matsuka K, Ito O, Matsuda H. Experimental study on alkaline skin injuries—periodic changes in subcutaneous tissue pH and the effects exerted by washing. Burns. 1993;19(4):320-323. doi:10.1016/0305-4179(93)90120-w
9. Davidson EC. The treatment of acid and alkali burns: an experimental study. Ann Surg. 1927;85(4):481-489. doi:10.1097/00000658-192704000-00001
10. Andrews K, Mowlavi A, Milner SM. The treatment of alkaline burns of the skin by neutralization. Plast Reconstr Surg. 2003;111(6):1918-1921. doi:10.1097/01.PRS.0000058953.16695.A7
11. Bizrah M, Yusuf A, Ahmad S. An update on chemical eye burns. Eye (Lond). 2019;33(9):1362-1377. doi:10.1038/s41433-019-0456-5
12. Milner SM. Skin grafting. In: Evans GRD, ed. Operative Plastic Surgery. 2nd ed. Oxford University Press; 2020:65-73.
13. Friedstat JS, Klein MB. Acute management of facial burns. Clinics in Plastic Surgery. 2009;36(4):653-660. doi: 10.1016/j.cps.2009.05.004
14. Foster K, Greenhalgh D, Gamelli RL, et al. Efficacy and safety of a fibrin sealant for adherence of autologous skin grafts to burn wounds: results of a phase 3 clinical study. J Burn Care Res. 2008;29(2):293-303. doi:10.1097/BCR.0b013e31816673f8