Cardiogenic Shock: A Systematic Review of Clinical Trials Registered With ClinicalTrials.gov
Abstract: Background. Despite a range of devices, medical interventions, and revascularization techniques utilized in cardiogenic shock (CS), there is a lack of evidence guiding management. We sought to characterize the contemporary trials through utilization of the ClinicalTrials.gov database. Methods. We investigated all phase II-IV interventional trials in the ClinicalTrials.gov database through June 29, 2019 that enrolled patients with CS. Published trials investigating medical interventions were evaluated for methodological quality using the Jadad scoring system. Results. The initial query yielded 28 registered studies, of which 28 directly studied CS through whole or subgroup analyses. Of these, five were withdrawn or terminated, while 13 were recruiting, not yet recruiting, or were of unknown recruitment status. The remaining 10 were published and had a median patient size of 69 patients and a median site size of 6. Of the published studies, all-cause mortality was the most common primary endpoint (60%), including composite endpoints that included mortality. The remaining endpoints examined surrogate hemodynamic parameters of cardiac function through echocardiography. The mean Jadad score of the published trials investigating pharmacological therapies was 2.42. Of the trials investigating device therapies or revascularization methods, all were randomized, parallel-arm studies that were open label. Conclusions. Modern trials vary from single center to multicenter and are small in size. The primary endpoints were clinical, focusing on mortality and restoration of cardiac output or cardiac index. Methodological quality varies in the trials focused on pharmacologic therapy. Trials with devices or revascularization do not employ blinding, but do employ randomization.
Key words: cardiogenic shock, circulatory support devices, guidelines
Cardiogenic shock (CS) has had numerous definitions in the literature but is broadly recognized as a low cardiac output state resulting in end-organ hypoperfusion. Despite advances in reperfusion techniques and hemodynamic support device implantation, there remains high morbidity and mortality for patients with CS. Acute myocardial infarction (AMI) remains the most common cause of CS and complicates approximately 5.7%-8.6% of patients with AMI.1-4 AMI complicated by CS confers a grave prognosis, and despite the reduction in mortality for AMI in recent decades, AMI with CS still results in a hospital mortality rate of 24.6%-51.0%.4-6
Currently, there is a wide knowledge gap that exists surrounding the optimal management of patients with CS. Cardiology guideline statements note potential areas of research, including defining the optimal timing and delivery of critical care treatment, efficacy and safety of treatment modalities, and optimal management of vasopressors and hemodynamic support devices.7 CS is inherently problematic to investigate, as informed consent is difficult to obtain in the setting of shock, critically ill patients are usually excluded from the studies, and randomization and blinding pose difficult challenges with intrinsic trial constraints.
To narrow these knowledge gaps, expanding patient inclusion criteria to increase the overall sample size, using CS registries, and additional systematic methods are needed. Understanding of the current flaws and strengths of the available trials may help lead to better-conducted studies in the future. The purpose of the present analysis was to perform a systematic review of the ongoing contemporary trials focusing on CS as registered on ClinicalTrials.gov.
Methods
Clinicaltrials.gov search. We identified CS trials registered through June 29, 2019 in the ClinicalTrials.gov database using the following search limits: study phase II-IV, interventional design, and an adult population >18 years of age. The PRISMA search algorithm is illustrated in Figure 1.
Publications and data extraction. Published manuscripts were identified by searching the title of the study, principal investigator (PI), and the ClinicalTrials.gov registry number unique to each individual study in PubMed. When publications were available, the following data were extracted for the published manuscripts: number of patients enrolled, number of clinical sites, regional location of study, publication year, trial design, blinding strategy, funding source, and primary endpoint. Published data were then compared with information available on ClinicalTrials.gov for verification. Primary endpoints were classified as clinical, surrogate, or safety. If the trials investigated more than one primary endpoint, both were cataloged. If ClinicalTrials.gov listed publications that were not queried by the search parameters above, those were also included in the analysis.
Ongoing and future trials. Trials were identified through the original ClinicalTrials.gov search. Published manuscripts were identified by the same methods as described above. When published manuscripts were not available, information was collated through available information on ClinicalTrials.gov.
Withdrawn and terminated trials. Trials were identified through the original ClinicalTrials.gov search. Published manuscripts were identified by the same methods as described above. When published manuscripts were not available, information was collated through available information on ClinicalTrials.gov.
Assessment of clinical trial quality. All published trials investigating pharmacologic therapies were evaluated for methodological quality by using the Jadad scoring system,8 which evaluates studies based on the following criteria: randomization, blinding strategy, and nature of withdrawals/dropouts. Two authors (AS and OS) independently scored each published trial that investigated pharmacologic interventions. Given the inherent challenges of blinding studies focused on device therapies or the timing of revascularization, we detailed each trial component rather than using the Jadad scoring system to assess methodological quality.
Statistical analysis. An assessment of the inter-relator concordance of Jadad scoring was performed using Lin’s concordance coefficient (ρc). Jadad scoring was applied solely to published trials that had detailed descriptions of randomization, blinding, and participant dropouts.
Results
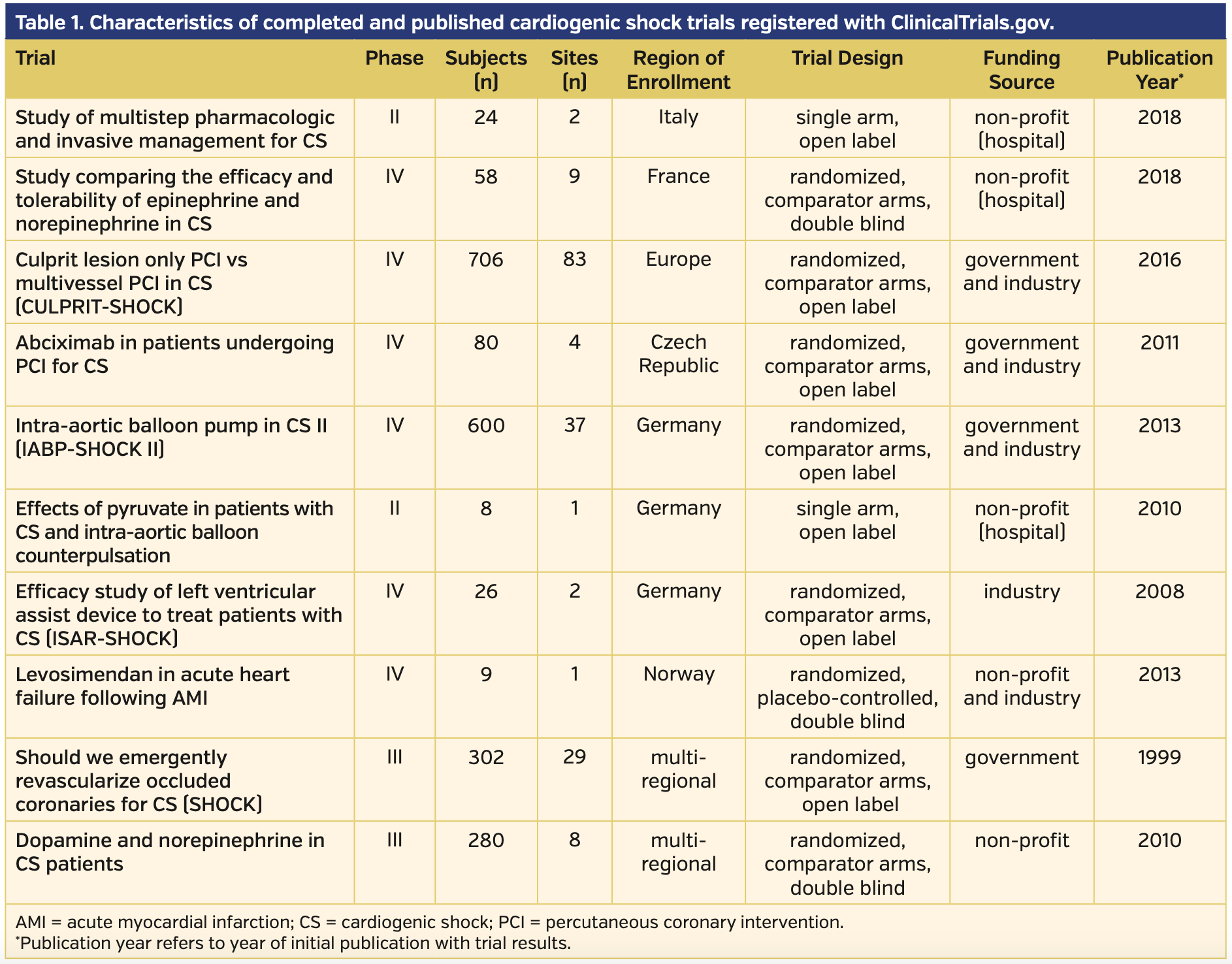
A total of 113,543 phase II to IV interventional trials were registered in ClinicalTrials.gov at the time of our search, of which 28 (0.02%) were registered to CS. After manual screening, five trials had been terminated or withdrawn and 13 were actively recruiting, not recruiting, or of unknown status. The remaining 10 studies were published (Figure 2).9-21 Published manuscripts were available for analyses comprising 2093 patients from 176 sites in total.
Etiologies and definitions of CS. There was heterogeneity in the etiologies of CS employed by the trials. Of the 10 published and completed trials, a total of 8 studied CS in the setting of AMI. The other 2 studies investigated all-cause CS, or CS secondary to new or worsening heart failure (HF). In the 8 studies investigating CS complicating AMI, five studies staged interventions following PCI in >92% of the study population, while 3 studies investigated either timing of PCI, number of vessels revascularized, or the use of the glycoprotein IIb/IIIa inhibitor, abciximab.
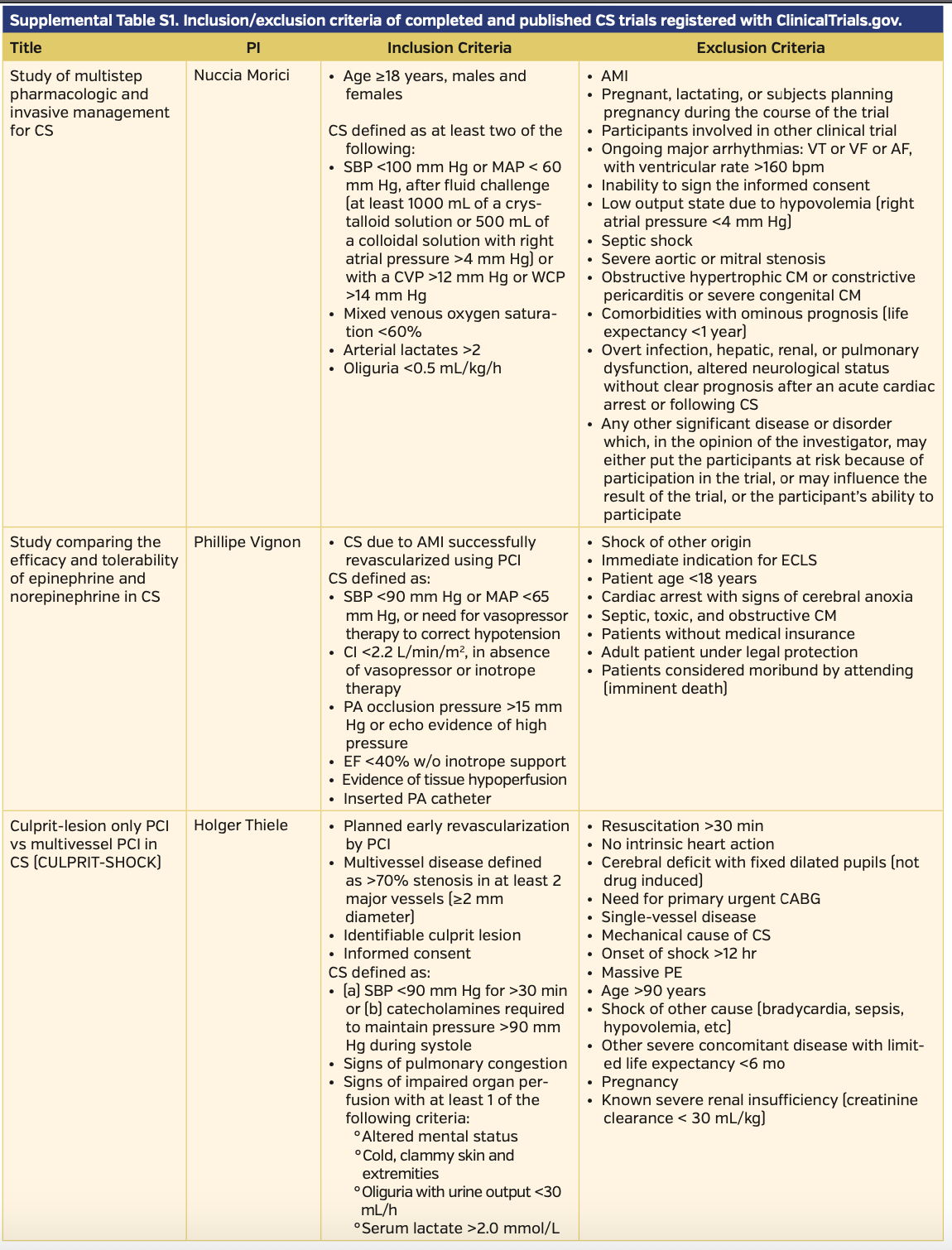
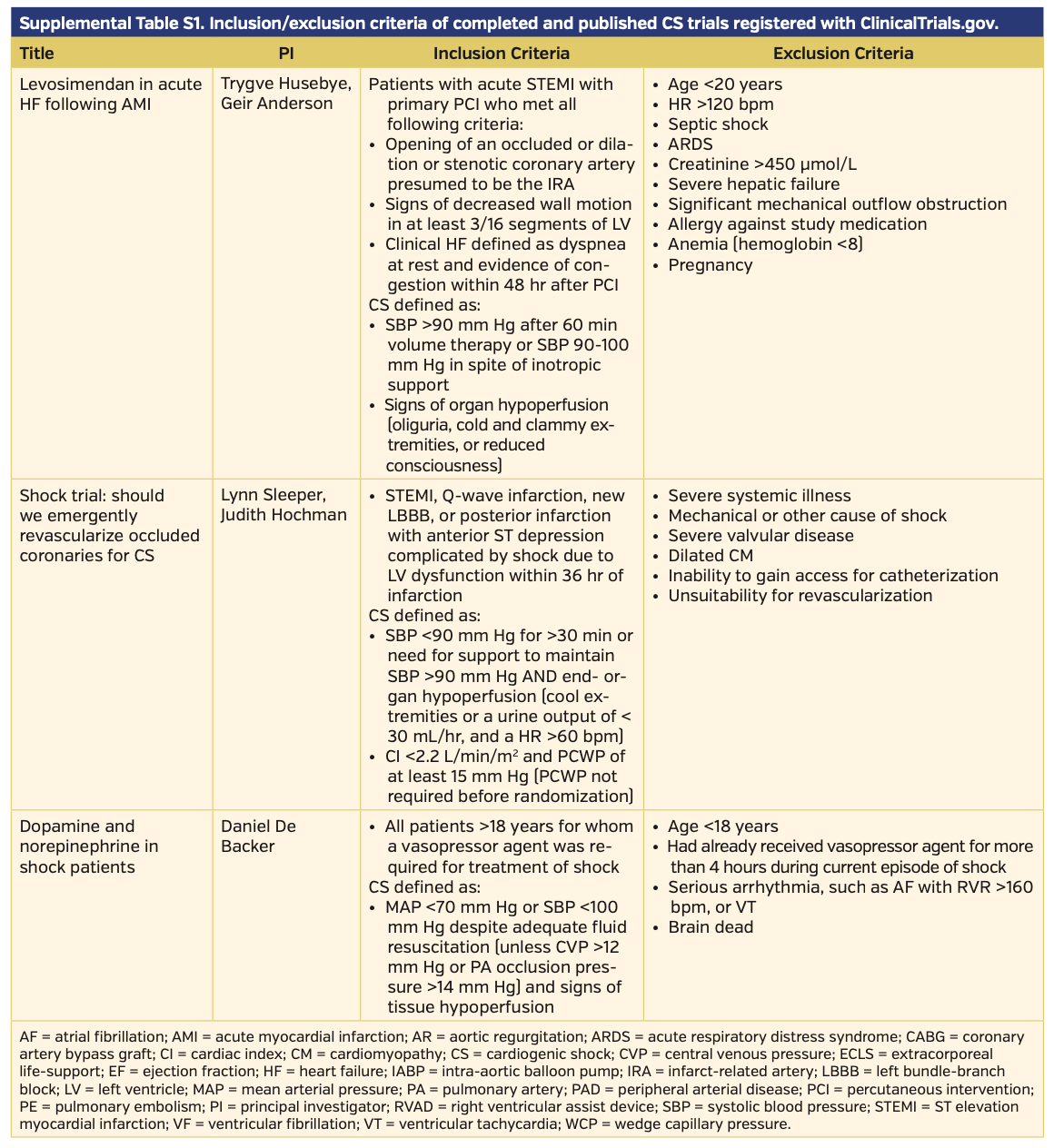
The definitions of CS varied slightly in their clinical and hemodynamic parameters among published trials. All published trials included hypotension as a clinical criterion. The trials most commonly employed systolic blood pressure (SBP) <90-100 mm Hg as the clinical definition of hypotension. A handful of studies used mean arterial pressure (MAP) <60-70 mm Hg as an adjunct or alternative sign of hypotension. Few studies required clinical signs of end-organ hypoperfusion, with the most common signs assessed being cold peripheral extremities, elevated lactate, oliguria with urine output <30 mL/hr, and altered mental status. Three studies utilized invasive cardiac monitoring with a pulmonary artery catheter to measure cardiac index or pulmonary capillary wedge pressures as an adjunct or diagnostic criterion. Complete CS definitions, as well as inclusion/exclusion criteria of completed and published trials, can be found in Supplemental Table S1.
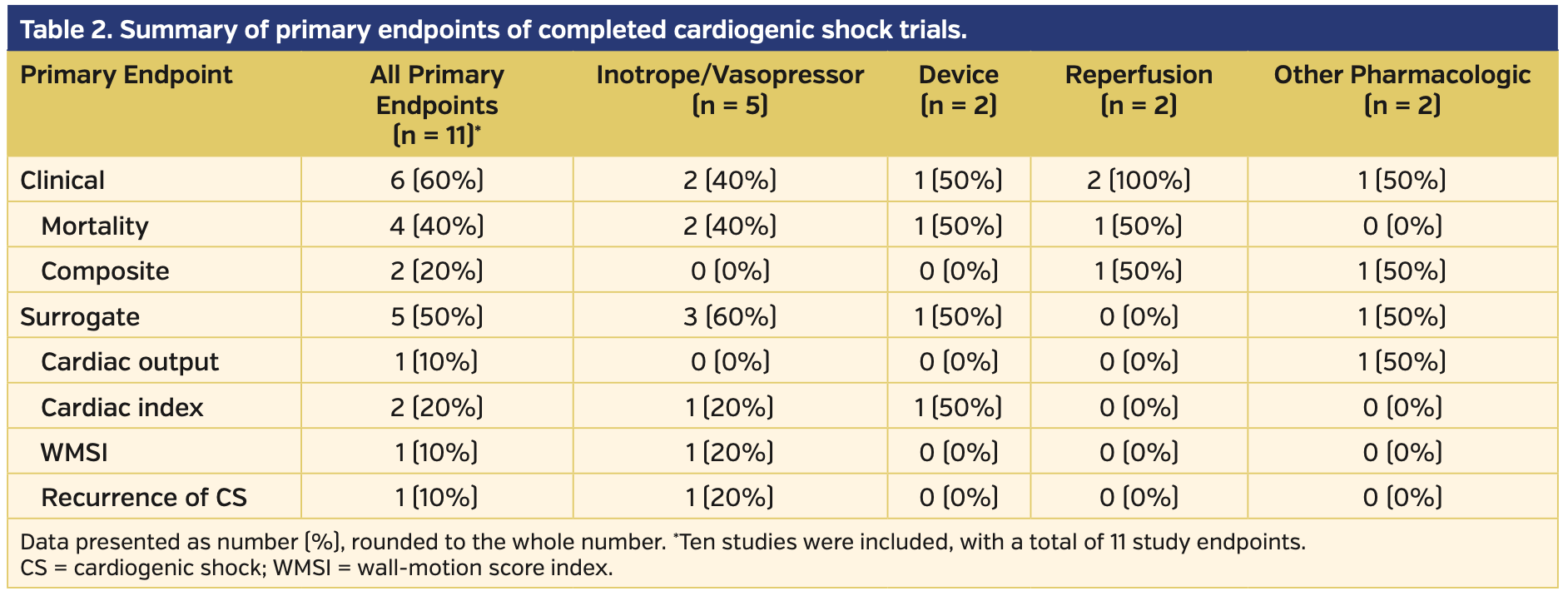
Published trials. Of the 10 published trials there was variation in patient volume, n = 8 to n = 706. Eighty percent of trials enrolled patients exclusively in Europe, and the other 2 trials were spread over multiple continents. The 10 published trials evaluated 11 primary endpoints, of which 6 were clinical endpoints, four were surrogate markers of hemodynamic stability, and the remaining endpoint evaluated was recurrence of CS. Six of the trials studied medical interventions, two trials investigated devices, and 2 investigated methods of revascularization. Of the medical interventions studied, four investigated vasopressor and/or inotropic medications, one studied an antiplatelet monoclonal antibody, abciximab, prior to revascularization, and 1 studied the use of sodium pyruvate. The trials investigating devices included the intra-aortic balloon pump and a left ventricular assist device (LVAD), the Impella 2.5 L catheter (Abiomed).
Mortality was the most common primary endpoint, analyzed in 6 trials either on mortality alone or included in a composite score. Temporal variations for the mortality endpoint varied between 38-60 days. The surrogate markers measured hemodynamic parameters through echocardiography, such as cardiac index, cardiac output, and wall-motion score index. A summary of published trials is provided in Table 1, and a summary of the primary endpoints investigated is provided in Table 2.
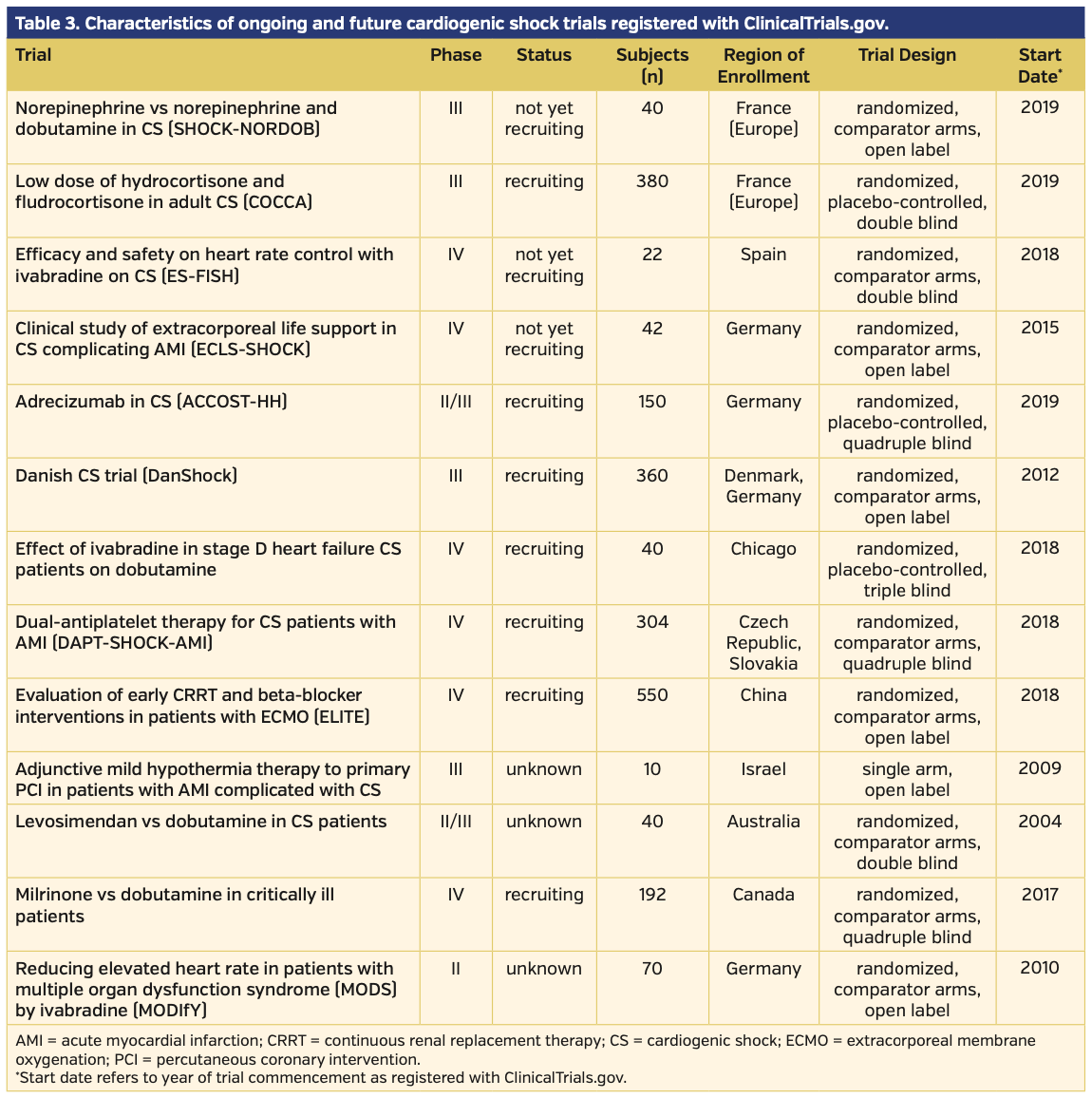
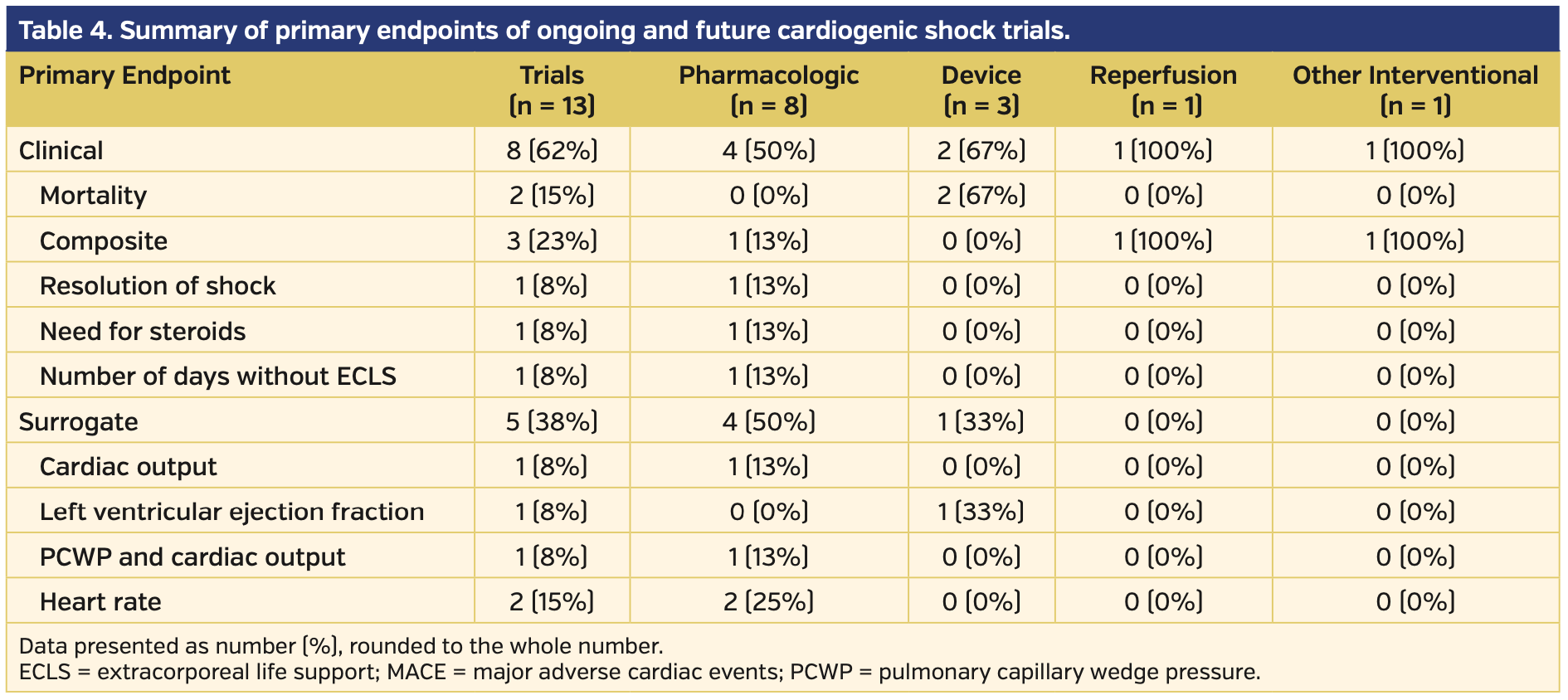
Future and ongoing trials. Of the 13 future and ongoing trials, eight investigated pharmacologic therapies alone, two investigated device therapies alone, one investigated a combination of pharmacologic and device therapy, one investigated pharmacologic therapy prior to PCI, and 1 investigated adjunctive hypothermia during and following PCI. All information unique to these studies was gathered from the ClinicalTrials.gov website. Seven of these 13 trials are actively recruiting, three are active but not recruiting, and 3 are of unknown status. There is an anticipated mean and median enrollment of 169 and 70 participants, respectively, for these trials. The trial phase ranged from II-IV. Of the 13 trials, six are registered as phase IV trials, four are registered as phase III, two are registered as combination phase II-III, and 1 is registered as phase II alone. All but 1 trial will employ randomization, with the remaining trial designed as a single-arm feasibility study. CS definitions listed per the ClinicalTrials.gov website unique to ongoing trials were variable. Four of the 13 trials directly investigated CS secondary to AMI. Other trials investigated all-cause CS or excluded some causes of CS (most frequently, CS secondary to arrhythmia). A summary of the ongoing and future trials is provided in Table 3, with primary endpoints provided in Table 4.
Withdrawn and terminated trials. Of the 5 withdrawn or terminated trials, four investigated pharmacologic therapies and 1 investigated the LVAD Impella (a mechanical cardiac support device). There was only 1 available publication unique to the terminated/withdrawn trials.22 The withdrawn trial aimed to investigate cyclosporine’s effect on patients with AMI complicated by CS, but was withdrawn before recruitment began. No available information was available for the reason of withdrawal. Three out of the 4 trials that were terminated were done so because of low enrollment. The remaining trial was terminated due to a prespecified futility analysis that was performed at 50% of anticipated enrollment. The trials that were terminated due to low enrollment comprised a small number of clinical sites ranging from n = 1 to n = 13. A summary of the withdrawn and terminated trials is provided in Table 5.
Assessing methodological quality in published pharmacologic trials. Trial quality for the 6 pharmacologic trials was evaluated with high concordance between the two authors (concordance coefficient, 0.982; 95% confidence interval [CI], 0.881-0.997). The mean Jadad score of these studies was 2.42 and ranged from low methodological quality of 0 to high methodological quality of 5. Three trials (50%) were graded as an average between authors of a Jadad score of <2, and 3 trials (50%) were graded ≥3. Two trials (33%) were graded a Jadad score of 5, the highest score possible.
Assessing methodological quality in device and revascularization trials. Of the 4 CS trials involving device or revascularization, all were randomized and all had two trial arms. In addition, all trials had comparator arms and were open label; no sham procedures were used in the comparator arms.
Discussion
We systematically characterized all 28 trials of CS registered with ClinicalTrials.gov, and independently analyzed 10 trials that were published for methodological quality. Our analysis identified several trial-level designs including: (1) the most common primary endpoint studied was all-cause mortality, and the second leading primary endpoint was a measure of hemodynamic stability; (2) the trials had varying number of participants and were generally small, with a median and mean of 69 and 209 subjects, respectively; (3) the trials had a varying number of clinical sites, with a median and mean of 6 and 17.6 respectively; (4) methodological quality of trials investigating CS was highly variable; and (5) trials of devices and revascularization methodology frequently employed randomization and comparator arms, but did not include blinding.
The majority of published trials focused on all-cause mortality as the primary endpoint, while the remaining trials evaluated parameters of hemodynamic stability. A variety of hemodynamic parameters were analyzed, including cardiac index, cardiac output, and wall-motion score index. An analysis of the SHOCK registry determined that cardiac power output was the highest predictor of mortality in patients with CS; however, none of the published trials utilized cardiac power output as a primary endpoint.23 Since CS is burdened with a high in-hospital mortality rate, endpoints incorporating or predicting all-cause mortality can be beneficial for assessing outcomes, and may be more appropriate than surrogate markers of hemodynamic stability alone.
Despite the rising incidence and lack of decline in mortality of CS, guidelines on management of CS remain difficult to define.24,25 While mortality has improved in the recent decades with the use of percutaneous coronary intervention and mechanical circulatory support, the mortality and morbidity rates for CS remain high. There is need for exploration of new device and drug therapies to improve outcomes. While not all clinical trials are registered with the ClinicalTrials.gov registry, it remains a unique tool with which to study current research of CS trials. Despite its strengths as a registry, it does not catalogue all trials, nor does it catalogue all publications related to registered trials. Because of this, we used PubMed as an adjunct resource. This approach is not all-encompassing in characterizing all CS research and data. However, it is a beginning to hopefully guide future research trial design and improve guideline recommendations.
CS remains an inherently difficult disease to study in clinical trials. There is urgent need for pharmacologic or device therapy to restore hemodynamic stability, and randomization and blinding pose challenges with intrinsic CS time management constraints. These difficulties are reflected in the termination of 3 CS clinical trials due to slow enrollment. Multicenter studies will allow for larger patient enrollment. Furthermore, there are slight variations in how CS is defined in trials. Standardizing definitions can aid in direct comparison and meta-analyses of CS clinical trials, and may assist in the difficulties of low enrollment.
Trial quality is variable among CS trials. Among the pharmacologic trials, there was heterogeneity in excellent and poor methodological quality designs. Two trials were single-arm studies, which is fundamentally a poor method to measure the effect of interventions. The other study that was deemed a poor methodological design was burdened by poor randomization according to date of admission, as well as lack of blinding when it was feasible. The trials deemed high-quality methodologies employed appropriate randomization through a randomized block design, and appropriate blinding of patients and care providers. Compared with the methodological quality of trials in other interventional therapies, the quality of CS trials underperforms.26,27 Among the studies investigating devices or revascularization, there was consistent use of proper randomization; however, all trials were open label due to the fundamental obstacles in blinding device or revascularization therapies.
While not the primary objective, two trials followed patients long term as a secondary endpoint to evaluate long-term clinical efficacy. There have been recent updates concerning two well-powered and methodologically sound trials. The SHOCK group followed patients long term and noted an increased 6-year survival rate in patients who underwent early revascularization compared with those who were assigned to medical stabilization first (6-year survival rates for survivors were 62.4% vs 44.4%, respectively).28 The IABP-SHOCK II group recently published long-term 6-year follow-up outcomes for 591 out of 600 patients and noted no difference in mortality between the intra-aortic balloon pump group and the control group (mortality rates were 66.3% and 67.0%, respectively).29 While in-hospital mortality for CS remains high, long-term mortality and morbidity rates of interventions employed in trials are crucial for assessing outcomes when patients leave the hospital.
Conclusion
We constructed a comprehensive review of CS studies registered within the ClinicalTrials.gov database. Our analysis identifies that methodological quality of CS trials is variable, possibly owing to the inherent difficulties in studying device therapies, as well as the difficulty in recruiting patients, as seen in the withdrawn or terminated trials. The primary endpoints of the completed trials were all clinical, with mortality the most common endpoint. Although CS will remain a difficult and complex condition to study, precise study design including appropriate blinding and randomization can advance evidence-based guidelines for CS. While the recruitment of patients with CS remains an active challenge, adequately powered trials are necessary in order to improve patient morbidity and mortality.
From the University of Texas Health Science Center at San Antonio, San Antonio, Texas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted October 15, 2019, provisional acceptance given October 18, 2019, final version accepted November 1, 2019.
Address for correspondence: Omar Sheikh, MD, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229. Email: sheikho@uthscsa.edu
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117:686-697.
- Fox KA, Anderson FA Jr, Dabbous OH, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart. 2007;93:177-182.
- Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294:448-454.
- Aissaoui N, Puymirat E, Tabone X, et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J 2012;33:2535-2543.
- Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-long trends (2001-2011) in the incidence and hospital death rates associated with the in-hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117-125.
- Davierwala PM, Leontyev S, Verevkin A, et al. Temporal trends in predictors of early and late mortality after emergency coronary artery bypass grafting for cardiogenic shock complicating acute myocardial infarction. Circulation. 2016;134:1224-1237.
- van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232-e268.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12.
- Morici N, Sacco A, Paino R, et al. Cardiogenic shock: how to overcome a clinical dilemma. Unmet needs in emergency medicine. Int J Cardiol. 2015;186:19-21.
- Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72:173-182.
- Thiele H, Desch S, Piek JJ, et al. Multivessel versus culprit lesion only percutaneous revascularization plus potential staged revascularization in patients with acute myocardial infarction complicated by cardiogenic shock: design and rationale of CULPRIT-SHOCK trial. Am Heart J. 2016;172:160-169.
- Quayyum Z, Briggs A, Robles-Zurita J, et al. Protocol for an economic evaluation of the randomised controlled trial of culprit lesion only PCI versus immediate multivessel PCI in acute myocardial infarction complicated by cardiogenic shock: CULPRIT-SHOCK trial. BMJ Open. 2017;7:e014849.
- Tousek P, Rokyta R, Tesarova J, et al. Routine upfront abciximab versus standard periprocedural therapy in patients undergoing primary percutaneous coronary intervention for cardiogenic shock: the PRAGUE-7 study. An open randomized multicentre study. Acute Card Care. 2011;13:116-122.
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638-1645.
- Fuernau G, Poss J, Denks D, et al. Fibroblast growth factor 23 in acute myocardial infarction complicated by cardiogenic shock: a biomarker substudy of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Crit Care. 2014;18:713.
- Jung C, Fuernau G, de Waha S, et al. Intra-aortic balloon counterpulsation and microcirculation in cardiogenic shock complicating myocardial infarction: an IABP-SHOCK II substudy. Clin Res Cardiol. 2015;104:679-687.
- Schillinger W, Hunlich M, Sossalla S, Hermann HP, Hasenfuss G. Intracoronary pyruvate in cardiogenic shock as an adjunctive therapy to catecholamines and intra-aortic balloon pump shows beneficial effects on hemodynamics. Clin Res Cardiol. 2011;100:433-438.
- Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584-1588.
- Husebye T, Eritsland J, Muller C, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail. 2013;15:565-572.
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779-789.
- Alexander JH, Reynolds HR, Stebbins AL, et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657-1666.
- Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44:340-348.
- Rab T, Ratanapo S, Kern KB, et al. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72:1972-1980.
- Jones TL, Nakamura K, McCabe JM. Cardiogenic shock: evolving definitions and future directions in management. Open Heart. 2019;6:e000960.
- Patel RB, Venkateswaran RV, Singh A, et al. The current landscape of atrial fibrillation and atrial flutter clinical trials: a report of 348 studies registered with ClinicalTrials.gov. JACC Clin Electrophysiol. 2018;4:944-954.
- Gautier I, Janiaud P, Rollet N, et al. Trends in the number and the quality of trial protocols involving children submitted to a French institutional review board. BMC Med Res Methodol. 2017;17:130.
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295:2511-2515.
- Thiele H, Zeymer U, Thelemann N, et al; for the IABPSHOCK II Trial (Intraaortic Balloon Pump in Cardiogenic Shock II) Investigators. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. Epub 2018 Nov 11.