Outcomes of Split-thickness Skin Grafting for Foot and Ankle Wounds in Patients With Peripheral Arterial Disease
In this study, the authors examine factors predictive of STSG healing in patients with PAD following vascular intervention, including the effect of non-inline flow via arterial-arterial and non-arterial collateralization.
Abstract
Introduction. Tissue ischemia resulting from arterial insufficiency is a major factor affecting lower extremity wound healing in patients with peripheral arterial disease (PAD). Accelerated wound closure with split-thickness skin grafting (STSG) provides a durable barrier to infection and can prevent limb loss. Published STSG outcomes data are minimal in the post endovascular intervention population. Objective. In this study, the authors examine factors predictive of STSG healing in patients with PAD following vascular intervention, including the effect of non-inline flow via arterial-arterial and non-arterial collateralization. Materials and Methods. Patients with PAD and wounds of the foot and ankle who underwent STSG between January 2014 and December 2016 were retrospectively reviewed. All patients received angiographic evaluation and endovascular or open revascularization where necessary. Effects of extremity revascularizations, STSG percent take, and amputation rate were evaluated. Results. Thirty-five patients with 47 wounds underwent STSG. There were 21 men and 14 women with a mean age of 64 ± 13 years. Revascularization was required in 23 patients (25 extremities) before STSG, with balloon angioplasty for tibial artery lesions as the most common revascularization. Patent pedal arch was present in 8 patients; 35 patients had an absent or incomplete pedal arch. Patients with a fully patent pedal arch healed at a significantly higher rate than those with an absent or incomplete pedal arch at 1 month (62.5% vs. 17.1%, P < .05). At 90-day follow-up, 9 of 35 (25.7%) patients with 9 of 47 (19.1%) wounds were lost to follow-up, leaving 18 of 38 (47.37%) wounds healed and 20 (52.63%) still open. Ultimately, 36 of 47 (76.60%) wounds healed and 6 major amputations in 6 patients were required at a mean 502 ± 342 days follow-up. Conclusions. These results suggest the importance of arterial-arterial connections such as the pedal arch to the healing potential of foot and ankle wounds after STSG in this high-risk patient population.
Introduction
About 8 million Americans over the age of 65 have peripheral arterial disease (PAD).1 Due to the ever-increasing prevalence of diabetes and chronic kidney disease and an aging population, multilevel PAD and chronic total occlusions have become more common.2,3 Critical limb ischemia (CLI) with advanced wound presentation is a major cause of nontraumatic lower extremity above-the-knee (AKA) and below-the-knee amputations (BKA). Such amputations are an expensive and catastrophic complication often leading to permanent disability and a concomitant decrease in functional status and quality of life.4
The goal for health care providers managing ischemic wounds is to expedite rapid and stable wound closure to create a durable protective barrier to further infection and limb loss. Wound closure can be achieved by conservative measures such as local wound care or by using split thickness grafting (STSG) and other surgical methods.5 Split-thickness skin grafting requires local control of infection and a vascularized wound bed for success and is a relatively simple procedure, but literature on wound closure rates after STSG is limited in patients with PAD.6-8
This paper reports the outcomes of using STSG for lower extremity wound closure in patients with PAD. Patients underwent preoperative vascular evaluation and revascularization as needed. Factors predictive of complete wound healing after STSG (defined as 100% take) at 30 and 90 days were analyzed and compared with those in patients with less than 100% STSG take or persistent open wounds.
Materials and Methods
A retrospective review was performed for all patients with lower extremity wounds and PAD who underwent STSG at a single institution (MedStar Georgetown University Hospital, Washington, DC) between January 2014 and December 2016. Patients with foot and ankle wounds with evidence of ischemia underwent angiographic vascular evaluation and revascularization by a co-author (CMK) and senior vascular surgeon at the Center for Wound Healing and Hyperbaric Medicine at MedStar Georgetown University Hospital. Patients without adequate perfusion or where revascularization (via endovascular or open revascularization) was not possible were excluded from this study. Patients with STSG over local flaps or free tissue transfer also were excluded, as were patients with inframalleolar interventions or with wound etiologies other than ischemia, such as postacute trauma or excision of malignancy. Patients needed to have at least 1 follow-up visit within 30 days of the STSG to be included in the study. All but 1 patient underwent preoperative catheter angiography of the lower extremity.
Foot and ankle wound perfusion was documented as per the lower extremity angiosome model,9,10 with the angiosome defined as a 3D block of tissue supplied by a specific source artery and drained by a specific vein.10 The foot can be divided into 6 main angiosomes arising from the posterior tibial artery (3 angiosomes), anterior tibial artery (1), and peroneal artery (2) (Figure11).10,11 The posterior tibial artery gives rise to a calcaneal branch that supplies the medial ankle and plantar heel, a medial plantar branch that irrigates the medial plantar instep, and a lateral plantar branch that feeds the lateral forefoot, plantar midfoot, and entire plantar forefoot. The anterior tibial artery continues on to the dorsum of the foot as the dorsalis pedis. The peroneal artery gives rise to a calcaneal branch that supplies the plantar heel and lateral ankle as well as an anterior branch that feeds the anterior upper ankle. The flow provided by the tibial vessels is interconnected between angiosomes by arterial-arterial connections. The pedal arch communicates the dorsal and plantar arterial flow, and the peroneal artery distal branches connect this vessel with the circulation from the anterior and posterior tibial arteries at the ankle and foot.
Ischemic wounds in this cohort were classified as directly revascularized (DR) or indirectly revascularized (IR). If the feeding artery supplying the angiosome of the wound was patent with inline flow, wounds were classified as DR. If direct inflow was not patent or could not be achieved by revascularization, wounds were classified as IR. Indirectly revascularized wounds were subdivided into (1) IR with the presence of arterial-arterial connections (IRAA) to supply the angiosome containing the wound, or (2) IR without patent arterial connections but indirect flow to the angiosome, with no named vessel supplying the angiosome of the wound.11 For example, revascularization of ischemic ulcers located at the dorsum of the foot fed by the patent dorsalis pedis artery was considered to be DR. If dorsal wounds were supplied by the plantar vessels via a patent pedal arch in the absence of a patent dorsalis pedis artery, these wounds were considered to be IRAA. Wounds on the dorsum of the foot without a patent pedal arch and without a patent dorsalis pedis artery were classified as IR without arterial connections.
A complete pedal arch was defined when both the dorsalis pedis artery and at least one of the plantar arteries were patent and directly connected to one another. An incomplete pedal arch was defined when the dorsalis pedis artery or one of the plantar arteries were patent but not connected to one another. An absent pedal arch was defined when neither the dorsalis pedis artery nor the plantar arteries were patent. In this case, circulation of the foot was established through collateral vessels.12 The decision to revascularize via endovascular or open intervention was based on wound location, size, successful wire crossing across diseased segments, and overall fitness to withstand surgical bypass based on comorbidities. Patients received STSG when perfusion was determined to be satisfactory and control of infection was achieved via systemic antibiotics and wound bed preparation with excisional debridement.
Split-thickness skin graft placement was performed as per the following STSG preparation protocol to achieve a granulating, clean wound bed. All patients received a minimum of 2 separate surgical debridements, the first several days prior to the grafting procedure and the other on the day of STSG immediately before skin grafting. Post-debridement cultures taken from the debridement several days prior to STSG were defined as culture 1. This debridement was the penultimate debridement patients underwent before STSG. After culture 1 revealed scant or minimal growth, the decision was made to proceed with STSG. Immediately before STSG, patients underwent a final debridement to prepare the wound bed for skin grafting. Cultures were taken both before and after this final debridement, after which the STSG was placed. Cultures taken predebridement were termed culture 2, and cultures taken after this debridement were referred to as culture 3. All cultures were taken in the operating room, with swabs of the wound bed obtained using the Levine technique.13 Predebridement cultures were taken prior to prepping and draping, and post-debridement cultures were taken intraoperatively immediately following debridement, irrigation, and redraping.
In the operating room, wounds were debrided excisionally by scalpel, rongours, or a hydrosurgical device (VERSAJET; Smith & Nephew, Fort Worth, TX). Following debridement, all wounds were irrigated with 3 L of normal saline. Drapes and gloves of all surgical team members in the operating room were changed after irrigation. A separate clean table with new surgical instrumentation was used following debridement. Once wound sites were deemed suitable for skin grafting, a dermatome (Zimmer Biomet, Warsaw, IN) was used to harvest the skin from the thigh at a thickness range of 0.010 in to 0.018 in (0.30 mm–0.45 mm). The graft then was meshed at a ratio of 1.5:1 or pie crusted and placed on the prepared wound bed. A silicone interface followed by negative pressure wound therapy or sponge tie-over bolster were used as a dressing depending on size, location, and surgeon preference. Graft take rate was assessed weekly with location-specific offloading and/or immobilization applied as needed. Total contact casting or controlled ankle motion (CAM) boots were used as appropriate. Debridement and skin grafting were performed per institutional guidelines by the 4 senior surgeons (JSS, KKE, CEA, PJK).
Patient demographic data, comorbidities, wound characteristics, lower extremity arteriogram findings, wound classification as per the angiosome anatomical model, and revascularization procedures were reviewed and documented. Graft takes at 30 and 90 days as well as ultimate wound outcomes, including major and minor amputation rates, were documented. Residual open wound size was used as a surrogate measure for graft take at 30 days, 90 days, and the last post-graft clinic visit day. Unless explicitly mentioned by the surgeon in the medical record, percent graft take was assessed by the Formula.
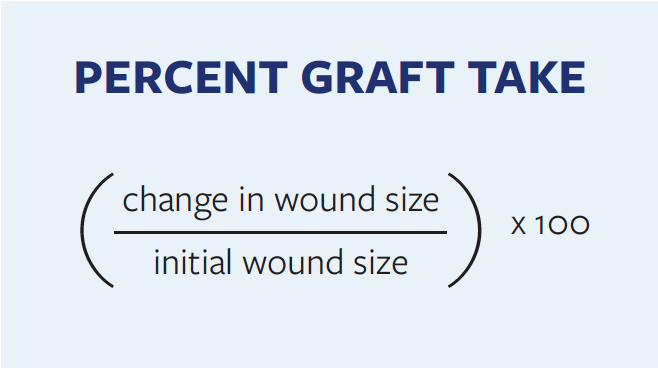
Wounds with 100% STSG take at 30 days were defined as healed. All foot reoperations, STSG revisions, and target extremity revascularization procedures were recorded. Major amputation was defined as an amputation above the ankle, and factors affecting healing outcomes were analyzed and compared.
Statistical analysis was performed using Statistical Analysis System software version 9.4 (SAS Institute Inc, Cary, NC). Continuous variables were described by means and standard deviations; median, first quartile, and third quartiles were reported where appropriate. The 2-sample t test and Wilcoxon rank sum test were used to compare distribution of continuous variables. Categorical variables were described by frequencies and percentages, with chi-square and Fisher’s exact tests used as appropriate to compare proportions of categorical variables. Multivariate logistic regression analysis was performed to determine the stated factors that affect the outcome of STSG, adjusting for variables found significant in the bivariate analysis. A successful outcome of STSG was defined as 100% take of the STSG. Take rate was based on change in initial wound size recorded or on explicit take rate stated in the patient charts. Statistical significance was defined as P < .05.
Results
Thirty-five patients with 47 wounds on 38 extremities met inclusion criteria for this retrospective study. There were 21 (60%) men and 14 (40%) women with a mean age of 64 ± 12.6 years (range, 39–89 years). Diabetes was present in 34 patients (97.1%), hypertension in 34 (97.1%), hyperlipidemia in 24 (68.6%), end-stage renal disease in 10 (28.6%), venous stasis in 8 (22.9%), and congestive heart failure (CHF) in 13 (37.1%) patients. Nineteen (54.3%) patients had a current or prior history of smoking. Mean hemoglobin A1c was 7.1 ± 1.8 (range, 4.2–14.2) and mean body mass index was 31.3 kg/m2 ± 7.1 kg/m2 (range, 19.5–47.9 kg/m2). At 30 days, 12 of 47 (25.5%) wounds were fully healed. Mean percent take of STSG at 30 days was 79.4% ± 28.3%; at 90 days, 18 of 47 (38.3%) wounds were fully healed.
Several factors affecting wound healing at 30 and 90 days were analyzed, including patient characteristics, wound parameters, complications, procedural (ie, angiogram) characteristics, wound classification, and post-STSG wound care characteristics. Clinical characteristics and wound parameters are shown in Table 1. Procedural factors and post-STSG care are presented in Table 2. Pedal arch patency was found to be significantly predictive of wound healing at 30 days, as 5 of 8 (62.5%) patients with a patent pedal arch achieved complete healing while only 6 of 35 (17.1%) patients without a patent pedal arch achieved complete healing (P = .01). Multivariate logistic regression analysis revealed no significant relationship between wound healing and patient comorbid conditions, plantar and nonplantar wounds, the presence of bacteria at different culture time points, or various methods of wound bed preparation, such as the application of Integra Dermal Regeneration Template (Integra LifeSciences Corp, Plainsboro NJ). Mean follow-up was 503 ± 338 days (range, 31–1189 days). During this period, 36 of 47 (76.6%) wounds achieved complete wound closure with a median time to healing of 54 days (Q1 = 31, Q3 = 142). The time to wound healing was 16.3 ± 25.4 weeks (median: 9, Q1 = 4, Q3 = 28) in the DR group, 23 of 25 (92%) wounds healed; 13.9 ± 20.7 weeks in the IRAA group (median: 7, Q1 = 5, Q3 = 13), 11 of 16 (68.75%) wounds healed; and 56.0 ± 72.1 weeks in the IR group (median: 56, Q1 = 5, Q3 = 107), and 2 of 6 (33.33%) wounds healed (P = .128).
Regarding additional procedures performed for limb salvage, after excluding the 6 wounds that resulted in amputation, 13 of 41 (31.7%) wounds required a mean 4.15 ± 2.82 additional procedures for limb salvage following STSG (median: 4, Q1 = 2, Q3 = 5). These included 28 surgical debridements, 7 angiographic interventions, 6 applications of a dermal regeneration template, 3 additional STSGs, 5 partial foot amputations, 1 xenograft placement, 1 free flap, and 3 tissue rearrangement procedures for wound closure.
Additional descriptive analysis was performed based on individual extremities to assess limb salvage rates. Twenty-five extremities in 23 patients underwent successful revascularization, resulting in a total of 34 wounds undergoing revascularization before STSG coverage. The most common intervention was balloon angioplasty for tibial lesions, which occurred in 14 (56%) extremities. Seven (28%) extremities had concomitant above-the-knee and below-the-knee interventions including stent and angioplasty of femoropopliteal segments. Four (16%) extremities with extensive arterial lesions with a favorable surgical risk profile underwent open bypass. Two (8%) had femoropopliteal bypass and 2 (8%) underwent popliteal to distal posterior tibial bypass. Two extremities (8%) did not achieve revascularization due to inability to cross lesions endovascularly and were not candidates for open revascularization due to severe comorbidities. Split-thickness skin graft was performed in these 2 extremities after the wounds showed evidence of granulation with local wound care.
Of the 35 patients, 13 did not receive revascularization before STSG. In 2 extremities, endovascular procedures were unsuccessful, and revascularization was not attempted in 5 other cases due to long-segment calcified total occlusions, which would make endovascular intervention high risk, as well as severe patient comorbidities prohibiting open surgical bypass. In another 6 cases, revascularization was not considered as the wounds were deemed to have adequate perfusion via IRAA.
Six of 38 (15.8%) extremities ultimately progressed to major amputation (5 BKA, 1 AKA) due to severe untreatable PAD. Two of these 6 patients underwent open bypass that subsequently occluded. Two of the 6 underwent endovascular revascularization of the femoropopliteal segment; these 2 wounds healed at 30 days but subsequently recurred due to worsening PAD with previously treated vessel segment occlusion. Two of the 6 other patients for whom revascularization was never an option required BKA. Mean time from STSG to major amputation was 559 ± 309 days (range, 140–1099 days). Congestive heart failure was present in 5 of 6 (83.3%) of the patients who required amputation in comparison with 10 of 35 (24.4%) patients with CHF who did not (P = .009). Other factors were not statistically significantly different between patients who received amputation and those who did not.
Discussion
Split-thickness skin grafting provides stable and durable wound closure. Outcomes of STSG have been reported in different patient populations such as patients with diabetes, wounds of multiple etiologies, and PAD-associated wounds.6,7 However, there is a paucity of literature describing the outcome of STSG for lower extremity wound coverage in patients following endovascular revascularization. This retrospective study examined time to wound healing as per wound perfusion rather than effects of revascularization. Thus, if the wound was adequately perfused, even via indirect arterial connections, direct inflow vessel reconstruction was not performed to avoid the substantial risk associated with intervening in long segment calcified tibial occlusions.
This study focused on factors affecting healing at 30 days, as complete wound healing within this time period suggests a clean and well-vascularized wound. Beyond 30 days post-STSG application, additional host factors may contribute to wound healing failure, including immunodeficiency that prolongs healing time or biomechanical issues resulting in wound reoccurrence.14 In this study group, 3 extremity wounds that initially healed recurred after 90 days due to deformity with Charcot arthropathy or trauma with subsequent severe infection resulting in major amputation.
At MedStar Georgetown University Hospital there is a high incidence of normal ankle-brachial index (ABI) in patients with atherosclerotic risk factors presenting with lower extremity wounds or gangrene. Similarly, research has shown that about 30% of patients with documented CLI have near normal, normal, or noncompressible ABI.15 Thus, it is the authors’ practice to proceed with catheter angiography in these patients to best evaluate the lower extremity vascular supply and afford the opportunity to improve perfusion via intervention. Literature related to patients with CLI has consistently reported that both endovascular and surgical therapies to improve perfusion to the distal extremity were superior to medical management alone.16,17 After diagnostic angiography, the decision not to revascularize was made based on either subjective assessment of adequate perfusion of the wound despite tibial and inframalleolar disease or on the fact that the patients were poor candidates for improving perfusion by revascularization.
The goal of angiosome-directed therapy is to improve perfusion to the area of the wound to facilitate wound healing.10,11 As such, it is essential to have a good understanding of direct and indirect revascularization including the role of patent arterial connections. These arterial connections are sometimes confused with collaterals, which are usually small-sized choke vessels rather than true arterial connections. These arterial connections can be identified by assessing wound perfusion by catheter angiogram and classified into the IRAA group or the IR group. These arterial connections can provide adequate perfusion to wounds in the absence of direct inline flow in patients for whom endovascular interventions are not possible due to disease severity. In the authors’ patient population, IRAA utilizing arterial connection to perfuse wounds resulted in better wound healing than direct revascularization. This may be explained by the finding that multiple patent tibial vessels were present in the IRAA group, whereas only 1 tibial vessel supplied the angiosome of the wound within in the DR group. The authors also found poor healing outcomes in patients with no patent tibial vessels to the foot, which is similar to findings by Faglia et al.18 Preoperative wound perfusion status according to the angiosome model as described earlier and supported by Varela et al10 can be applied to predict wound healing after STSG and counseling patients for realistic outcomes.12 Overall, 26.8% (11/41) of wounds that achieved perfusion via DR and IRAA had healed at 30 days. This number improved to 52.9% (18/34) by 90 days of follow-up. The improved healing rate seen at 90 days may be explained by the time it takes for complete epithelialization after STSG in patients with PAD. Restoring blood flow to the angiosome at the wound location was the main goal of performing angiography and revascularization. There was observed improved healing among wounds that had perfusion via DR and IRAA compared with IR, but this did not reach statistical significance, which may be due to the small sample size.
A complete pedal arch was found to be the most significant factor predictive of wound healing in this study. Recently, Troisi et al12 also have reported the positive impact of a complete pedal arch on time to healing, limb salvage, and survival in their cohort of 137 patients with diabetes. Their patients did not receive STSG, but rather mostly foot amputation and debridement. The authors of the current study found similar positive effects of a complete pedal arch on wound healing after STSG at 30 days. Further, there was an incomplete or absent pedal arch in 82.9% (29/35) of patients who failed to heal after STSG. A complete patent pedal arch was present in 41.7% (5/12) of patients who ultimately healed, but only in 8.6% (3/35) who did not heal after STSG.
A correlation between PAD, wound infection, and wound healing has been reported by Prompers et al,14 who demonstrated worse healing rates in patients with PAD than in those without PAD (64% vs. 84%, P < .001) at 1-year follow-up.15 In the current study, no significant difference in healing outcomes was found between wounds with or without bacteria on semiquantitative culture, and no significant difference in healing outcomes was found based on bacterial species cultured. This may be due to low bacterial load, with growth being reported as mostly rare and scant.
Khan et al19 conducted a study reporting major amputations in patients with patent endovascular-treated arterial segments (PETAS). The authors reported a 38% limb loss rate in extremities with patent open bypass and 80% in extremities with PETAS (P = .001). Risk factors for amputation in patients with PETAS were extensive tissue loss, recurrent infection, and failure to reverse ischemia. In the current study cohort, among endovascularly treated patients, 2 required major amputations. One patient experienced stent occlusion at 6 months and the other at 16 months, with no further options for revascularization. There were no major amputations in patients with PETAS. These results may be due to the authors’ practice of an aggressive approach to control infection, early wound coverage by STSG, and early vascular evaluation and revascularization. It should, however, be noted that progression of atherosclerosis after multiple successful vascular interventions sometimes results in amputations, especially when patients present with extensive gangrene and life-threatening infection.
Complete wound healing defined as 100% STSG take at 30 days in this cohort was 12 of 47 (25.5%). Increasing wound size requiring STSG was associated with inferior healing at 30 days. Wounds healed at 30 days were a mean 20.3 cm2 ± 22.2 cm2 in size compared with a mean size of 41.8 cm2 ± 40.8 cm2 in patients whose wounds had not completely healed. Similar findings have been reported in the literature.6 The low 30-day healing rate found in the present study could be related to multiple host factors such as diabetes, nutritional status, compliance to therapy, and PAD, each of which may slow wound healing independently.20 Among the 35 wounds that did not achieve 100% STSG take at 30 days, 24 (68.6%) wounds did eventually heal.
Limitations
This study is limited by many factors, including a small sample size. Only patients with a minimum follow-up of 30 days following application STSG were included in this cohort. Four patients had no follow-up beyond 6 months. Further, the authors did not have noninvasive perfusion assessments such as ABI measurements in all patients, so they were unable to quantitatively document improvements in perfusion in patients who underwent successful revascularization.
Conclusions
A patent pedal arch is very important for foot and ankle wound healing after STSG and can be used to predict success of STSG. As this study shows, a multidisciplinary team approach to achieve a clean, revascularized wound bed for foot and ankle wounds and early coverage with STSG can help in preventing major above-the-ankle amputations in patients with PAD.
Acknowledgments
Authors: Iram Naz, MD1; Elliot T. Walters, MD1; David E. Janhofer, BS2; Morgan M. Penzler, BS2; Eshetu A. Tefera, MS1; Karen Kim Evans, MD1; John S. Steinberg, DPM1; Christopher E. Attinger, MD1; Cameron M. Akbari, MD1; and Paul J. Kim, DPM, MS1,3
Affiliations: 1Center for Wound Healing and Hyperbaric Medicine, Department of Plastic Surgery, MedStar Georgetown University Hospital, Washington, DC; 2Georgetown University School of Medicine, Washington, DC; and 3Department of Plastic Surgery, University of Texas Southwestern Medical Center, Dallas, TX
Correspondence: Paul J. Kim, DPM, MS, Professor, Department of Plastic Surgery, Department of Orthopedic Surgery, University of Texas Southwestern WA4 238, Outpatient Building, 1801 Inwood Road, Dallas, TX 75390; Paul.Kim@UTSouthwestern.edu
Disclosure: Data were presented at the 2018 Cardiovascular Research Technologies Annual Meeting (Washington, DC). The authors disclose no financial or other conflicts of interest. During the time of this study, Dr. Kim was affiliated with MedStar Georgetown University Hospital.
















