ADVERTISEMENT
Effect of Glycolic Extract of Dillenia indica L. Combined With Microcurrent Stimulation on Experimental Lesions in Wistar Rats
Abstract: This study evaluated the wound healing activity of a glycolic extract of Dillenia indica (GED) prepared from the mature fruits of the plant applied alone or in combination with microcurrent stimulation to skin wounds surgically induced on the back of Wistar rats. Methods. The animals were randomly divided into six groups: (A) negative control group; (B) group receiving microcurrent application (MC; [10 mA/2 mins]); (C) group treated with GED; (D) group treated with an emulsion containing GED; (E) group treated with GED and MC, and (F) group treated with the emulsion containing GED and MC. Tissue samples were obtained 2, 6, and 10 days after injury and underwent structural and morphometric analysis. Results. There were observed differences in wound healing among the various treatments when compared to the control group. The combination of microcurrent plus extract or microcurrent plus emulsion containing GED was advantageous in all of the studied parameters (P < 0.05) when compared to the other groups with positive effects seen regarding newly formed tissue, number of fibroblasts, and number of newly formed blood vessels. The morphometric data confirmed the structural findings. Conclusion. Microcurrent application alone or combined with GED exerted significant effects on wound healing in this experimental model. This was probably due to the efficacy of microcurrent application since the extract alone did not significantly accelerate the healing process.D indica fruit extract most likely participates in the wound healing process as a result of its anti-inflammatory properties. Dillenia indica L. (family Dilleniaceae) originated in tropical Asia and has acclimated in Brazil for more than a century. The plant has a thick trunk and a fissured bark surface. The branches have leaves that are concentrated on the terminal region. The flowers are large and white in color and bloom between December and April. The fruits that appear between April and August are large, fleshy, and greenish-yellow in color and contain small, flattened seeds surrounded by a gelatinous substance.1–4 D indica is used as an antipyretic and cardiotonic drug and for the treatment of rheumatoid arthritis and anti-inflammatory processes.5–8 Munit et al9 identified the presence of triterpenes and flavonoids in a phytochemical study of the crude extract of D indica leaves. Abdille et al2 observed that the extract of D indica fruit contains significant amounts of phenolic compounds with expressive antioxidant activity. Flavonoids, tannins, and other phenolic substances are constituents of plants with antioxidant activity, mainly by acting as radical scavengers of oxygen. The presence of flavonoids in phytotherapeutic agents has been shown to favor the wound healing process in experimental models.10,11 Wound healing is a complex biological process that occurs in response to tissue damage due to trauma or surgical procedures. The wound healing process can be divided into three phases: inflammatory, proliferative, and remodeling. Technological advances facilitated the emergence of a wide variety of wound healing treatments. The application of low amperage electrical stimuli has been shown to modify the healing process in living organisms, especially factors that delay or impair this process.12–16 Several investigators have studied the effects of electrical stimulation using different amplitudes and frequencies and observed modifications in the cellular and tissue responses in experimentally induced wounds.17–19 Stimulation of live cells with low-intensity electrical currents directly affects the membrane potential and is associated with changes in ion gradients across the cell membrane causing an increase in the synthesis of ATP followed by increased protein synthesis.12,20,21 The objective of this study was to investigate the effects of a glycolic extract of Dillenia indica L. fruits and an emulsion containing this extract, either with or without microcurrent stimulation, on the healing of surgically-induced wounds in Wistar rats.
Wound healing is a complex biological process that occurs in response to tissue damage due to trauma or surgical procedures. The wound healing process can be divided into three phases: inflammatory, proliferative, and remodeling. Technological advances facilitated the emergence of a wide variety of wound healing treatments. The application of low amperage electrical stimuli has been shown to modify the healing process in living organisms, especially factors that delay or impair this process.12–16 Several investigators have studied the effects of electrical stimulation using different amplitudes and frequencies and observed modifications in the cellular and tissue responses in experimentally induced wounds.17–19 Stimulation of live cells with low-intensity electrical currents directly affects the membrane potential and is associated with changes in ion gradients across the cell membrane causing an increase in the synthesis of ATP followed by increased protein synthesis.12,20,21 The objective of this study was to investigate the effects of a glycolic extract of Dillenia indica L. fruits and an emulsion containing this extract, either with or without microcurrent stimulation, on the healing of surgically-induced wounds in Wistar rats.
Methods
Mature fruits of D indica L. were collected in February 2009 on the Pinhal Farm in Limeira (São Paulo, Brazil) in the morning. Material was collected from the branches of the same tree for deposition of a voucher specimen, which was deposited by Vinícius Castro Souza, Curator of the Herbarium of the Department of Biological Sciences, ESALQ-USP, Piracicaba Campus (São Paulo) under the number ESA 55549.  Preparation of plant extracts. Mature fruits were collected and dried in an oven under circulating air at 45˚C for weight stabilization. The fruits were then ground in a knife mill. The extract was obtained by turbo extraction of 50 g of the sample in 500 mL 70% (w/w) alcohol for 15 minutes followed by filtration in a rotary evaporator under reduced pressure at a maximum temperature of 40˚C until complete elimination of the organic solvent. The turbo extraction technique does not allow the temperature to exceed 40˚C. This procedure was performed because it was considered relatively harmless regarding the extraction of different components. The sample was then lyophilized until all water was removed. For the physicochemical quality control tests, 10 g of mature fruit was dried at room temperature in the dark. Later, 1.0 g this material was subjected to infrared heat (110˚C) for 1 hour and was weighed afterward. This procedure was performed every hour until the weight did not vary more than 0.25%. Values are expressed as percentage (w/w). The average of determinations was three.22,23 For microbiological analysis of the lyophilized D indica extract, total microorganism count, and counts of the pathogens Salmonella sp, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa were determined according to the methods of Migliato et al24 and Pinto et al.25 Phytochemical screening. Preliminary phytochemical analysis of the D indica glycolic extract was performed in triplicate according to Simões et al26 and Harbone.27 A portion of 100 mg of the extract was used for each reaction. The presence of flavonoids was determined using 1% aluminum chloride solution in methanol, concentrated hydrochloric acid, magnesium turnings, and potassium hydroxide solution. Anthraquinones were analyzed by the Borntrager reaction and saponins by the observation of persistent and abundant foam production. To analyze tannins, the lyophilized extract was dissolved in water and tannins were identified by reaction with 2.5% gelatin, 1% iron salts, and 10% lead acetate. Total alkaloids were assayed using the reagents of Dragendorff, Bouchardat, Mayer, and Bertrand. Preparation of an emulsion containing the glycolic extract of Dillenia indica. The lyophilized D indica extract was solubilized in propyleneglycol:water (1:1) and incorporated into an emulsion containing the following: Butylated hydroxytoluene (BHT [0.05%]), Ethylenediamine tetraacetic acid (EDTA [0.1%]), Lanaxan® (2%), Polawax® (14%), Phenonip® (0.5%), propyleneglycol (3%), D indica glycolic extract (5%), and distilled water qsp (30%). Animals. Male Wistar rats (Rattus norvegicus) weighing 250 g–350 g were housed individually in cages at a constant temperature (23˚C ± 2˚C). The rats had free access to food and water and were subjected to a 12-hour light/dark cycle. The average weight and behavior during this experiment did not differ significantly at the end of the study.
Preparation of plant extracts. Mature fruits were collected and dried in an oven under circulating air at 45˚C for weight stabilization. The fruits were then ground in a knife mill. The extract was obtained by turbo extraction of 50 g of the sample in 500 mL 70% (w/w) alcohol for 15 minutes followed by filtration in a rotary evaporator under reduced pressure at a maximum temperature of 40˚C until complete elimination of the organic solvent. The turbo extraction technique does not allow the temperature to exceed 40˚C. This procedure was performed because it was considered relatively harmless regarding the extraction of different components. The sample was then lyophilized until all water was removed. For the physicochemical quality control tests, 10 g of mature fruit was dried at room temperature in the dark. Later, 1.0 g this material was subjected to infrared heat (110˚C) for 1 hour and was weighed afterward. This procedure was performed every hour until the weight did not vary more than 0.25%. Values are expressed as percentage (w/w). The average of determinations was three.22,23 For microbiological analysis of the lyophilized D indica extract, total microorganism count, and counts of the pathogens Salmonella sp, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa were determined according to the methods of Migliato et al24 and Pinto et al.25 Phytochemical screening. Preliminary phytochemical analysis of the D indica glycolic extract was performed in triplicate according to Simões et al26 and Harbone.27 A portion of 100 mg of the extract was used for each reaction. The presence of flavonoids was determined using 1% aluminum chloride solution in methanol, concentrated hydrochloric acid, magnesium turnings, and potassium hydroxide solution. Anthraquinones were analyzed by the Borntrager reaction and saponins by the observation of persistent and abundant foam production. To analyze tannins, the lyophilized extract was dissolved in water and tannins were identified by reaction with 2.5% gelatin, 1% iron salts, and 10% lead acetate. Total alkaloids were assayed using the reagents of Dragendorff, Bouchardat, Mayer, and Bertrand. Preparation of an emulsion containing the glycolic extract of Dillenia indica. The lyophilized D indica extract was solubilized in propyleneglycol:water (1:1) and incorporated into an emulsion containing the following: Butylated hydroxytoluene (BHT [0.05%]), Ethylenediamine tetraacetic acid (EDTA [0.1%]), Lanaxan® (2%), Polawax® (14%), Phenonip® (0.5%), propyleneglycol (3%), D indica glycolic extract (5%), and distilled water qsp (30%). Animals. Male Wistar rats (Rattus norvegicus) weighing 250 g–350 g were housed individually in cages at a constant temperature (23˚C ± 2˚C). The rats had free access to food and water and were subjected to a 12-hour light/dark cycle. The average weight and behavior during this experiment did not differ significantly at the end of the study.  Linear incision wound model. A trichotomy was performed on the back of the animal 48 hours before surgical intervention. After local asepsis with 0.4% chlorhexidine digluconate, the animals were anesthetized by intraperitoneal injection of xylazine hydrochloride (20 mg/kg body weight) and ketamine hydrochloride (50 mg/kg). After the position was marked with a dermographic pen and pachymeter, a 2-cm long and 0.2-cm deep surgical incision was made in the craniocaudal direction. The incision was not sutured. In view of the similar genetic background of the animals28,29 and according to the Ethics Committee of Uniararas (protocol number 809/2006), nine animals were used per group: (A) negative control group receiving sterile saline; (B) group receiving microcurrent application (MC; [10 mA/2 min]); (C) group treated with the glycolic extract of D indica (GED); (D) group treated with the emulsion containing GED; (E) group treated with the GED and MC (10 mA/2 min), and (F) group treated with the emulsion containing GED and MC (10 mA/2 min), according to the protocol of Mendonça et al.16 A transcutaneous electrical stimulator (Physiotonus Microcurrent, Bioset, Rio Claro, São Paulo, Brazil) was used for electrical stimulation. The device was set to microgalvanic-continuous mode with the intensity at 10 mA/2, frequency 0.3 Hz, and was used for 2 minutes. The applications were performed using two metal electrodes with a spherical tip (10 mm) positioned on the wound. The treatments were started 24 hours after surgical intervention and were continued daily for 10 days. Collection and preparation of wound samples for structural analysis. At days 2, 6, and 10 after the injury, three animals in each group were killed under anesthesia. The total area (approximately 120 mm2–160 mm2) of the wound was removed and submitted for structural and morphometric analysis. Each sample was removed and fixed in 10% formalin in Millonig buffer (pH 7.4) for 24 hours at room temperature. Next, the specimens were washed in buffer and processed for embedding in Paraplast®. Longitudinal sections (7 µm) were stained with hematoxylin & eosin for routine histology and with picrosirius-hematoxylin in order to view collagen fibers. The specimens were examined and documented using a Leica DM 2000 photomicroscope at the Laboratory of Micromorphology, Hermínio Ometto University Center, Uniararas. Morphometric analysis. Cross-sections of the mid-region of the experimental wound were used for the determination of the following morphometric parameters: tissue repair area (x103 µm2), total number of cells (fibroblastic and inflammatory cells [n/103 µm2]), number of newly formed blood vessels (n/103 µm2), and thickness of the regenerating epithelium (µm). For this purpose, three samples were randomly selected among the sections obtained. All images were captured and digitalized using a Leica DM 2000 photomicroscope. The measurements were made on the digitalized images using the Leica Image Measure™ and Sigma Scan Pro 6.0™ programs. The results were compared by ANOVA and Tukey’s post-hoc test with the level of significance set at 5%.30 The results were entered into spreadsheets (Biostat for Windows).
Linear incision wound model. A trichotomy was performed on the back of the animal 48 hours before surgical intervention. After local asepsis with 0.4% chlorhexidine digluconate, the animals were anesthetized by intraperitoneal injection of xylazine hydrochloride (20 mg/kg body weight) and ketamine hydrochloride (50 mg/kg). After the position was marked with a dermographic pen and pachymeter, a 2-cm long and 0.2-cm deep surgical incision was made in the craniocaudal direction. The incision was not sutured. In view of the similar genetic background of the animals28,29 and according to the Ethics Committee of Uniararas (protocol number 809/2006), nine animals were used per group: (A) negative control group receiving sterile saline; (B) group receiving microcurrent application (MC; [10 mA/2 min]); (C) group treated with the glycolic extract of D indica (GED); (D) group treated with the emulsion containing GED; (E) group treated with the GED and MC (10 mA/2 min), and (F) group treated with the emulsion containing GED and MC (10 mA/2 min), according to the protocol of Mendonça et al.16 A transcutaneous electrical stimulator (Physiotonus Microcurrent, Bioset, Rio Claro, São Paulo, Brazil) was used for electrical stimulation. The device was set to microgalvanic-continuous mode with the intensity at 10 mA/2, frequency 0.3 Hz, and was used for 2 minutes. The applications were performed using two metal electrodes with a spherical tip (10 mm) positioned on the wound. The treatments were started 24 hours after surgical intervention and were continued daily for 10 days. Collection and preparation of wound samples for structural analysis. At days 2, 6, and 10 after the injury, three animals in each group were killed under anesthesia. The total area (approximately 120 mm2–160 mm2) of the wound was removed and submitted for structural and morphometric analysis. Each sample was removed and fixed in 10% formalin in Millonig buffer (pH 7.4) for 24 hours at room temperature. Next, the specimens were washed in buffer and processed for embedding in Paraplast®. Longitudinal sections (7 µm) were stained with hematoxylin & eosin for routine histology and with picrosirius-hematoxylin in order to view collagen fibers. The specimens were examined and documented using a Leica DM 2000 photomicroscope at the Laboratory of Micromorphology, Hermínio Ometto University Center, Uniararas. Morphometric analysis. Cross-sections of the mid-region of the experimental wound were used for the determination of the following morphometric parameters: tissue repair area (x103 µm2), total number of cells (fibroblastic and inflammatory cells [n/103 µm2]), number of newly formed blood vessels (n/103 µm2), and thickness of the regenerating epithelium (µm). For this purpose, three samples were randomly selected among the sections obtained. All images were captured and digitalized using a Leica DM 2000 photomicroscope. The measurements were made on the digitalized images using the Leica Image Measure™ and Sigma Scan Pro 6.0™ programs. The results were compared by ANOVA and Tukey’s post-hoc test with the level of significance set at 5%.30 The results were entered into spreadsheets (Biostat for Windows).
Results
Quality assurance is an important factor to be considered in the production of medications, cosmetics, and phytotherapeutic agents from the planning to the eventual release of the product to the consumer. Therefore, physicochemical (Table 1) and microbiological quality control tests of the D indica glycolic extract were performed. 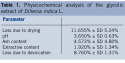 Analysis of total microorganism count showed no growth of viable fungal or bacterial colonies. None of the four pathogens investigated (Salmonella sp, E coli, S aureus, and P aeruginosa) was detected in the lyophilized extract. Preliminary phytochemical analysis of the glycolic extract of D indica fruits showed the presence of flavonoids, saponins, anthraquinones, and tannins; alkaloids were not detected.
Analysis of total microorganism count showed no growth of viable fungal or bacterial colonies. None of the four pathogens investigated (Salmonella sp, E coli, S aureus, and P aeruginosa) was detected in the lyophilized extract. Preliminary phytochemical analysis of the glycolic extract of D indica fruits showed the presence of flavonoids, saponins, anthraquinones, and tannins; alkaloids were not detected.  Structural and morphometric analysis of wound repair. Tissue repair was studied in the different groups by comparing inflammatory (leukocytosis, hemorrhage, and exudate) and proliferative processes (fibroblastic hyperplasia, epithelization, and angiogenesis), and tissue reorganization. Temporal differences in tissue repair were observed among the different treatments. In the control group (group A), the proliferative phase was observed on day 6 after injury and tissue reorganization was detected on day 10. In the group receiving MC (group B), the size of the tissue repair area and the total number of cells were higher than in the control group on day 6 of treatment. On day 10, the wound area was completely re-epithelialized with the observation of dermis filled with fibrous tissue, reorganized collagen fibers, and compacted fibril elements (Figures 1, 2). The findings obtained for the group treated with GED (group C) were similar to those observed for the control group in terms of the repair of epidermis and dermis for samples collected on days 6 and 10. A significant increase in the tissue repair area and the total number of cells was observed on days 6 and 10 after experimental injury in the group simultaneously receiving MC and GED (group E) when compared to the other groups. In this group, the dermal wound area was filled with fibrous tissue on day 10 and the collagen fibers were reorganized and compacted, findings indicating tissue repair similar to that observed in animals receiving only MC. No significant difference in the repair of the lining epithelium was observed during application of different treatments (Figures 1, 2).
Structural and morphometric analysis of wound repair. Tissue repair was studied in the different groups by comparing inflammatory (leukocytosis, hemorrhage, and exudate) and proliferative processes (fibroblastic hyperplasia, epithelization, and angiogenesis), and tissue reorganization. Temporal differences in tissue repair were observed among the different treatments. In the control group (group A), the proliferative phase was observed on day 6 after injury and tissue reorganization was detected on day 10. In the group receiving MC (group B), the size of the tissue repair area and the total number of cells were higher than in the control group on day 6 of treatment. On day 10, the wound area was completely re-epithelialized with the observation of dermis filled with fibrous tissue, reorganized collagen fibers, and compacted fibril elements (Figures 1, 2). The findings obtained for the group treated with GED (group C) were similar to those observed for the control group in terms of the repair of epidermis and dermis for samples collected on days 6 and 10. A significant increase in the tissue repair area and the total number of cells was observed on days 6 and 10 after experimental injury in the group simultaneously receiving MC and GED (group E) when compared to the other groups. In this group, the dermal wound area was filled with fibrous tissue on day 10 and the collagen fibers were reorganized and compacted, findings indicating tissue repair similar to that observed in animals receiving only MC. No significant difference in the repair of the lining epithelium was observed during application of different treatments (Figures 1, 2). 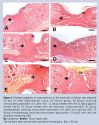 In the group treated with the emulsion containing GED (group D), the tissue repair area and total number of cells on days 6 and 10 after injury were similar to those of the control group. On day 10, the epidermis was completely repaired (Figures 3, 4). A significant increase in tissue repair area and the total number of cells was observed on days 6 and 10 in the group simultaneously receiving MC and the emulsion containing GEC (group F) when compared to group D. However, the collagen fibers of the dermis were poorly compacted.
In the group treated with the emulsion containing GED (group D), the tissue repair area and total number of cells on days 6 and 10 after injury were similar to those of the control group. On day 10, the epidermis was completely repaired (Figures 3, 4). A significant increase in tissue repair area and the total number of cells was observed on days 6 and 10 in the group simultaneously receiving MC and the emulsion containing GEC (group F) when compared to group D. However, the collagen fibers of the dermis were poorly compacted.  A significantly larger number of newly formed vessels per tissue area (103 µm2) were observed in groups B, E, and F (Figure 5). Epithelial thickness in the tissue repair area did not differ among groups when compared to control (Figure 6).
A significantly larger number of newly formed vessels per tissue area (103 µm2) were observed in groups B, E, and F (Figure 5). Epithelial thickness in the tissue repair area did not differ among groups when compared to control (Figure 6). 
Discussion
Phytochemical analysis of the glycolic extract of D indica fruits identified the presence of flavonoids, among other components. These compounds and their derivatives are known to increase vascularization and to delay the process of cell necrosis due to their anti-inflammatory, antifungal, and antioxidant properties.31,32 Studies have shown that most of the antioxidant activity of plant extracts is the result of compounds, such as flavonoids, tannins, isoflavones, flavones, anthocyanins, catechin, and other phenolic compounds.33,34 Flavonoids are especially important in the tissue repair process because of their astringent and antimicrobiological properties.6,35 The same properties can be attributed to tannins, which were identified in the present study in the glycolic extract of D indica . According to Jorge Neto et al36 and Bedi and Shenefelt,37 tannins precipitate proteins in damaged tissues forming a protective lining that favors repair and reduces wound permeability and exudation. Suntar et al38 reported that an aqueous extract of Colutea cilicica (Boiss. & Bal. fruit) was effective during the inflammatory and proliferative phases of the wound healing process in rats. The authors identified flavonoids in the C cilicica extract and suggested that these substances participate in the healing process together with other phytochemical components of the plant.  In the present study, although flavonoids were identified in the D indica extract, no significant effects on the acceleration of tissue repair were observed when the extract or the emulsion containing the extract was applied alone to skin wounds induced on the back of rats. Although the efficacy of flavonoids in the acceleration of the tissue repair process has been reported by different investigators,31,32 the present results indicate that the presence of these compounds in the D indica extract did not have this effect on the healing process. Analysis of the glycolic extract of D indica fruit did not reveal the presence of alkaloids. Similarly, Shome et al6 characterizing the phytochemical composition of hexane, chloroform, ethanol, and aqueous extracts of D indica fruits, demonstrated the presence of triterpenes and the absence of alkaloids in all extracts. Yeshwante et al39 observed significant anti-inflammatory activity of a methanol extract of D indica leaves on edema induced in rats and mice. The authors suggested that this process is the result of the inhibition of prostaglandin biosynthesis, confirming the use of D indica in folk medicine. In the present study, application of the D indica extract or of the emulsion containing the extract to skin wounds surgically induced in rats did not significantly accelerate the tissue repair process. Positive effects were only observed when microcurrent stimulation was applied simultaneously. These findings suggest that the extract of D indica fruit possesses the same properties as the leaf extract studied by Yeshwante et al,39 which showed efficacy in treating the effects of inflammatory processes, but did not accelerate wound healing. Microcurrent application was found to be effective in terms of the parameters analyzed, with positive effects on the area of newly formed tissue, number of fibroblasts and number of newly formed blood vessels, but not on epithelial thickness, when compared to the control group and to the groups receiving only the D indica extract and the emulsion containing the extract. These findings agree with those reported in different studies demonstrating that low-intensity electrical currents stimulate wound healing.17–19 Becker17 reported that electrical currents are present in all biological systems and may promote repair and growth after injury. According to this author, a specific injury stimulus induces another repair stimulus. The author also demonstrated that the membrane electrical potential is altered in injured tissues. The “injury signal” gradually decreases in parallel to the repair process and ceases when the latter is complete. The voltage peaks immediately after injury and gradually decreases as the wound heals, a fact leading to the concept that current flows may be defective in chronic wounds and that the application of electrical currents to wounds may stimulate healing.15,40 Biedeback41 proposed that transmembrane currents open voltage-controlled calcium channels in fibroblasts, inducing ATP resynthesis, activation of protein kinase mechanisms to synthesize new cellular protein, and DNA replication necessary for mitotic cell division. Electrical microcurrent has been used in the treatment of chronic wounds.42–44 Lee et al45 using a 100 nA current 3 µA in the treatment of chronic wounds and ulcers associated with chronic diseases and found that the application of such currents supposedly provides electrons to tissues and saturated free radicals, facilitating tissue repair. Mendonça et al16 suggested that microcurrent application to tissue injuries might be used as a coadjuvant to accelerate the healing process. Variations in cell metabolism, as well as fibroblast proliferation, neovascularization, and collagen deposition in the wound area have been observed after microcurrent application.16,46 The combined application of a microcurrent and the D indica extract or emulsion containing the extract was advantageous in terms of all parameters studied when compared to the control group and to either treatment alone. The simultaneous application of physical and phytotherapeutic agents to wounds has been shown to be effective in both skin repair and reduction of the inflammatory process. The use of electrical current on transdermal facilitating the transfer of various substances is known in the literature as iontophoresis—a noninvasive technique that ensures the penetration levels of higher concentrations of therapeutic substances when compared to passive diffusion. In iontophoresis, the current is widely used since it generates a unidirectional flow of electrons and constant during the application, triggering the desired therapeutic effects.47–49 Maia-Filho et al50 investigated the effects of simultaneous application of ultrasound and Aloe vera gel on an experimental model of induction of tendinitis in rats and demonstrated that this type of treatment is effective in terms of both skin repair and reduction of the inflammatory process. Also Soares51 demonstrated that the combination of antioxidant agents and photodynamnic or low-amperage electrical therapy accelerates wound healing in experimental wounds in rats. In addition, the simultaneous application of Aloe vera and microcurrent was effective in the treatment of open wounds potentiating wound healing in Wistar rats.16 Similar effects were observed in the present study. The combination of the D indica glycolic extract or emulsion containing the extract and microcurrent stimulation exerted significant effects on the repair area, total number of cells, and total number of newly formed vessels in the wound area. However, these alterations were not significant when the extract was applied alone. This fact suggests that the positive effects on the acceleration of tissue repair were due to the action of microcurrent stimulation, which has been shown to be effective in accelerating wound healing.15,16 However, the D indica fruit extract probably participates in the wound healing process as a result of its anti-inflammatory properties.
In the present study, although flavonoids were identified in the D indica extract, no significant effects on the acceleration of tissue repair were observed when the extract or the emulsion containing the extract was applied alone to skin wounds induced on the back of rats. Although the efficacy of flavonoids in the acceleration of the tissue repair process has been reported by different investigators,31,32 the present results indicate that the presence of these compounds in the D indica extract did not have this effect on the healing process. Analysis of the glycolic extract of D indica fruit did not reveal the presence of alkaloids. Similarly, Shome et al6 characterizing the phytochemical composition of hexane, chloroform, ethanol, and aqueous extracts of D indica fruits, demonstrated the presence of triterpenes and the absence of alkaloids in all extracts. Yeshwante et al39 observed significant anti-inflammatory activity of a methanol extract of D indica leaves on edema induced in rats and mice. The authors suggested that this process is the result of the inhibition of prostaglandin biosynthesis, confirming the use of D indica in folk medicine. In the present study, application of the D indica extract or of the emulsion containing the extract to skin wounds surgically induced in rats did not significantly accelerate the tissue repair process. Positive effects were only observed when microcurrent stimulation was applied simultaneously. These findings suggest that the extract of D indica fruit possesses the same properties as the leaf extract studied by Yeshwante et al,39 which showed efficacy in treating the effects of inflammatory processes, but did not accelerate wound healing. Microcurrent application was found to be effective in terms of the parameters analyzed, with positive effects on the area of newly formed tissue, number of fibroblasts and number of newly formed blood vessels, but not on epithelial thickness, when compared to the control group and to the groups receiving only the D indica extract and the emulsion containing the extract. These findings agree with those reported in different studies demonstrating that low-intensity electrical currents stimulate wound healing.17–19 Becker17 reported that electrical currents are present in all biological systems and may promote repair and growth after injury. According to this author, a specific injury stimulus induces another repair stimulus. The author also demonstrated that the membrane electrical potential is altered in injured tissues. The “injury signal” gradually decreases in parallel to the repair process and ceases when the latter is complete. The voltage peaks immediately after injury and gradually decreases as the wound heals, a fact leading to the concept that current flows may be defective in chronic wounds and that the application of electrical currents to wounds may stimulate healing.15,40 Biedeback41 proposed that transmembrane currents open voltage-controlled calcium channels in fibroblasts, inducing ATP resynthesis, activation of protein kinase mechanisms to synthesize new cellular protein, and DNA replication necessary for mitotic cell division. Electrical microcurrent has been used in the treatment of chronic wounds.42–44 Lee et al45 using a 100 nA current 3 µA in the treatment of chronic wounds and ulcers associated with chronic diseases and found that the application of such currents supposedly provides electrons to tissues and saturated free radicals, facilitating tissue repair. Mendonça et al16 suggested that microcurrent application to tissue injuries might be used as a coadjuvant to accelerate the healing process. Variations in cell metabolism, as well as fibroblast proliferation, neovascularization, and collagen deposition in the wound area have been observed after microcurrent application.16,46 The combined application of a microcurrent and the D indica extract or emulsion containing the extract was advantageous in terms of all parameters studied when compared to the control group and to either treatment alone. The simultaneous application of physical and phytotherapeutic agents to wounds has been shown to be effective in both skin repair and reduction of the inflammatory process. The use of electrical current on transdermal facilitating the transfer of various substances is known in the literature as iontophoresis—a noninvasive technique that ensures the penetration levels of higher concentrations of therapeutic substances when compared to passive diffusion. In iontophoresis, the current is widely used since it generates a unidirectional flow of electrons and constant during the application, triggering the desired therapeutic effects.47–49 Maia-Filho et al50 investigated the effects of simultaneous application of ultrasound and Aloe vera gel on an experimental model of induction of tendinitis in rats and demonstrated that this type of treatment is effective in terms of both skin repair and reduction of the inflammatory process. Also Soares51 demonstrated that the combination of antioxidant agents and photodynamnic or low-amperage electrical therapy accelerates wound healing in experimental wounds in rats. In addition, the simultaneous application of Aloe vera and microcurrent was effective in the treatment of open wounds potentiating wound healing in Wistar rats.16 Similar effects were observed in the present study. The combination of the D indica glycolic extract or emulsion containing the extract and microcurrent stimulation exerted significant effects on the repair area, total number of cells, and total number of newly formed vessels in the wound area. However, these alterations were not significant when the extract was applied alone. This fact suggests that the positive effects on the acceleration of tissue repair were due to the action of microcurrent stimulation, which has been shown to be effective in accelerating wound healing.15,16 However, the D indica fruit extract probably participates in the wound healing process as a result of its anti-inflammatory properties.
Conclusion
The present results demonstrated that the D indica glycolic extract or the emulsion containing the extract was not effective in accelerating the tissue repair process in skin wounds surgically induced in Wistar rats. However, microcurrent application alone or combined with glycolic extract exerted significant effects on wound healing in the experimental model. This finding was probably due to the efficacy of microcurrent stimulation since this treatment alone accelerated the healing process.
References
1. Alzugaray D, Alzugaray K. Enciclopédia de Plantas Brasileiras. São Paulo, Brazil: 3rd ed. 1988. 2. Abdille MH, Singh RP, Jayaprakasha GK, Jena BS. Antioxidant activity of the extracts from Dillenia indica fruits. Food Chemistry. 2005;90:891–896. 3. Lorenzi, H, Souza, HM, Torres, MAV, Bacher, LB. Árvores Exóticas no Brasil madeireiras, ornamentais e exóticas. Nova Odessa, SP: Instituto Plantarum; 2003. 4. Barroso, GM, Morim, MP, Peixoto, AL, Ichaso, CLF. Frutos e sementes: morfologia aplicada à sistemática de dicotiledôneas. Viçosa: UFV. 1999. 5. Shome U, Khanna KR, Sharma PH. Pharmacognostic studies on Dillenia indica Linn. I. Leaf. Proc Indian Acad Sci. 1979;88:35–48. 6. Shome U, Khanna KR, Sharma, PH. Pharmacognostic studies on Dillenia indica Linn. II. Fruit and seed. Proc. Indian Acad Sci. 1980;89:91–104. 7. Yeshwante, SB, Juvekar, AR, Nagmoti, DM, et al. Anti-inflammatory activity of methanolic extracts of Dillenia indica L. leaves. J Young Pharm. 2009;1(1):63–66. 8. Zauli-Nascimento RC, Pereira MA, Cardoso LGV, Silva JMSF, Carvalho JCT, Fiorini JE. Atividade antimicrobiana e determinação da concentração inibitória e concentração bactericida mínima de Dillenia indica L. (Flor de abril). In: XX Reunião Anual da FeSBe - Federação de Sociedades de Biologia Experimental. Águas de Lindóia-SP; 2005. 9. Munit A, Tareq SM, Apu AS, Basak D, Islam MS. Isolation and identification of compounds from the le Dillenia indica Linn. Bangladesh Pharma J. 2010;13(1):1–7. 10. Quettier-Deleu C, Gressier B, Vasseur J, et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum oench) hulls and flour. J Ethnopharmacol. 2000;72:35–42. 11. Peruchi, CMS, Acevedo, RA, Franco, SL. Efecto del propoleos en la cicatrización de lesions subcuaneas inducidas en el dorso del ratones, estudo histológico. Revista de La /facultad de Odontologia (Santiago, Chile). 2001;19:23–36. 12. Cheng N, Van Hoff H, Bocks E. The effect of electric currents on ATP generation, protein synthesis, and membrane transport in rat skin. Clin Orthop Relat Res. 1982;17:264–72. 13. Cheng K, Goldman RJ. Electric fields and proliferation in a dermal wound model: cell cycle kinetics. Bioelectromagnetics. 1998;19(2):68–74. 14. Houghton PE, Kincaid CB, Lovell M, Campbell KE, Keast DH, Woodbury MG, Harris KA. Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys Ther. 2003;83(1):17–28. 15. Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiment, and clinical trials. Int J Low Extrem Wounds. 2005;4(1):23–44. 16. Mendonça FAS, Passarini Jr JR, Esquisatto MAM, Mendonça JS, Franchini CC, Santos GMT. 2009. Effects of the application of Aloe vera (L.) and microcurrent on the healing of wounds surgically induced in Wistar rats (Rattus norvegicus). Acta Cir Bras. 2009;24:150–155. 17. Becker R, Selden G. The Body Electric. New York, NY; William Morrow and Co.: 1985. 18. Basset CA. Beneficial effects of electromagnetic-fields. J Cell Biochem. 1993;51:387–393. 19. Bayat M, Asgari-Moghadam Z, Maroufi M, Rezaie FS, Rakhshan M. Experimental wound healing using microamperage electrical stimulation in rabbits. J Rehabil Res Dev. 2006;43:219–228. 20. Santos VNS, Ferreira LM, Horibe EK, Duarte IS. Electric microcurrent in the restoration of the skin undergone a trichloroacetic acid peeling in rats. Acta Cir Bras. 2004;19:466–469. 21. Valle KKR, Reis LL, Bouvent JJ, Shida CS. Efeito da aplicação de microcorrente elétrica na restauração de pele de ratos exposta à ação de radicais livres. 21˚ CBEB. 2008;247–249. 22. Farmacopéia Brasileira. 4th ed. São Paulo, Brazil. Ed Atheneu; 2000. 23. Mello JCP, Petrovick PR. Quality control of Baccharis trimera (Less) DC (Asteraceae) hydroalcoholic extracts. Acta Farm Bonaerense. 2000;19:211–215. 24. Migliato KF, Moreira RRD, Mello JCP, Sacramento LVS, Corrêa MA, Salgado HRN. Controle da qualidade do fruto de Syzygium cumini (L.) Skeels. Braz J Pharmacogn. 2007;17:94–101. 25. Pinto TJA, Kaneko TM, Ohara MT. Controle Biológico de Qualidade de Produtos Farmacêuticos, Correlatos e Cosméticos. 2nd ed. São Paulo, Brazil: Atheneu; 2003. 26. Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz la, Petrovick PR. Farmacognosia: da Planta ao Medicamento. 5th ed. Porto Alegre: Editora da UFRGS; 2003. 27. Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 2nd ed. London: Chapman and Hill; 1998. 28. National Institutes of Health. Guide for the Care and Use of Laboratory Animals. NIH Publication. 1996;82–23. 29. Gill TJ, Smith GJ, Wissler RW. The rats as an experimental animal. Science. 1989;245(4915):269–276. 30. Miller RG. Simultaneous Statistical Inference. 2nd ed. New York, NY: Springer Verlag; 1981. 31. Santos SC, Mello JCP. In: Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, eds. Farmacognosia: da Planta ao Medicamento. 5th ed. Porto Alegre: Editora da UFRGS; 2003. 32. Nayak SB, Sandifer S, Maxwell A. Evaluation of the wound healing activity of ethanolic extract of Morinda citrifolia L. leaf. Avid Based Compl Alter Med. 2009;6:351–356. 33. Chalone MP, Hopi AI, Volutella HJ, et al. Antioxidant activity of plant extracts containing phenolics compounds. J Agric Food Chem. 1999;47(10):3954–3962. 34. Addison L, Goody JS, Ferrier LAM, Stedman JR, Brandi MGL. Proparacaine e caracterização de extratos glicólicos enriquecidos em taninos a partir das cascas de Stryphnodendron adstringens (Mart.) Coville (Barbatimão). Rev Bras Farmacol. 2002;12:7–34. 35. Pesin I, Koca U, Keles H, Kupeli Akkol E. Wound healing activity of Rubus sanctus Schreber (Rosaceae): preclinical study in animal models. Evid Based Complement Altern Med. 2009 Sept 15. [Epub ahead of print]. 36. Jorge Neto J, Fracasso JF, Neves MCLC, Santos LE, Banuth VL. Tratamento de úlcera varicosa e lesões de pele com Calendula officinalis e/ou com Stryphnodendron barbatiman (vellozo) martius. Ver Cienc Farm. 1996;17:181–186. 37. Bedi MK, Shenefelt PD. Herbal therapy in dermatology. Arch Dermatol. 2002;138:232–242. Ketylin F. Migliato, BSPS, PhD is from the 1University of Central Paulista, São Carlos, SP, Brazil and the Graduate Program of Pharmaceutical Sciences, Paulista State University, Araraquara, SP, Brazil; Mateus A. Chiosini, BSPS, Fernanda A. Mendonça, BSPS, PhD, Marcelo A. Esquisatto, PhD, and Hérida R. Salgado, BSPS, PhD are from the Graduate Program of Pharmaceutical Sciences, Paulista State University, Araraquara, SP, Brazil; Gláucia M.T. Santos, PhD is from the Graduate Program of Biomedical Sciences, Herminio Ometto University Center, Araras, SP, Brazil Address correspondence to: Gláucia M.T. Santos, PhD Hermínio Ometto University Center – UNARARAS Graduate Program of Pharmaceutical Sciences Av. Dr. Maximiliano Baruto, 500 Jd. Universitário 13607339 Araras, SP, Brazil Email: glauciasantos@uniararas.br

















