Developing and Evaluating Outcomes of an Evidence-based Protocol for the Treatment of Osteomyelitis in Stage IV Pressure Ulcers
Abstract
Osteomyelitis affects up to 32% of full-thickness pressure ulcers and increases treatment costs and the risk of systemic complications. Current diagnosis and treatment practices are variable. A literature and retrospective chart review, using a wound electronic medical record (WEMR), were conducted to develop an evidence-based protocol of care for treatment of osteomyelitis in pressure ulcers and to evaluate outcomes of care.
The seven steps in the protocol of care include: 1) acknowledgment of osteomyelitis risk in patients with Stage IV pressure ulcers, 2) clinical evaluation for local or systemic signs of infection upon initial presentation, 3) radiographic evaluation (magnetic resonance imaging or bone scan), 4) surgical debridement to remove all nonviable tissue and/or scarred and infected bone, 5) obtaining pathology reports from sterile bone biopsy and deep microbial cultures, 6) targeted systemic antimicrobial therapy, and 7) tissue reconstruction following resolution of infection. WEMR data review (177 patients) identified 50 patients with osteomyelitis (prevalence 28%). Of those, 41 underwent 87 bone debridements for osteomyelitis. Eight (20%) patients experienced complications related to treatment. Average time to discharge following debridement was 4.3 ± 5.7 days and 76% of wounds with more than two consecutive WEMR entries showed a decrease in area at their final visit. The outcomes observed are encouraging and the WEMR facilitates implementation and evaluation of the treatment protocol. Ongoing data acquisition will help assess outcomes and refine the current management protocol and should improve diagnosis and care.
With 1.3 to 3 million cases diagnosed annually in the US,1 and average charges reaching $37,800,2 pressure ulcers present a serious medical problem for hospitalized and/or bed-bound patients. Pressure ulcers have an estimated prevalence as high as 26% among hospitalized patients,3 53% among nursing home patients,4 and 39% among patients with spinal cord injuries.5 Pressure ulcers are associated with underlying physiologic impairments6-8 and can be difficult to heal even when appropriately treated.
Pressure ulcers increase the length of hospital stay, risk of nosocomial infection, and treatment costs.9 Evidence shows that pressure ulcers are associated with increased mortality rates10 and have been reported as a cause of death in more than 104,000 persons in the US annually.11 Septicemia was an underlying or contributing cause in 39.7% of all pressure ulcer-associated deaths.11
Stage IV pressure ulcers are particularly morbid. As much as 54% of Stage IV pressure ulcers require multiple hospital admissions12 and approximately 95% do not heal within 8 weeks.13 The 6-month mortality rate of patients with Stage IV ulcers can be as high as is 68.9%.14
 Osteomyelitis is characterized by a mixture of inflammatory cells (including neutrophils, lymphocytes, and plasma cells), fibrosis, bone necrosis, and new bone formation.When diagnosed in Stage IV pressure ulcers, osteomyelitis is associated with nonhealing wounds,15 surgical flap complications,16,17 and an increased length of hospitalization.16 Osteomyelitis may develop within the first 2 weeks of pressure ulcer formation and despite treatment may require amputation in lower extremity cases.18 The prevalence of osteomyelitis in full-thickness pressure ulcers ranges from 17%19 to 32%18 and in a prospective histologic and pathologic studies20 of bone cultures, the average cost of treatment was found to be $59,600. Despite the high prevalence of osteomyelitis, no large randomized controlled studies have been conducted with antibiotics nor have specific guidelines been promulgated.
Osteomyelitis is characterized by a mixture of inflammatory cells (including neutrophils, lymphocytes, and plasma cells), fibrosis, bone necrosis, and new bone formation.When diagnosed in Stage IV pressure ulcers, osteomyelitis is associated with nonhealing wounds,15 surgical flap complications,16,17 and an increased length of hospitalization.16 Osteomyelitis may develop within the first 2 weeks of pressure ulcer formation and despite treatment may require amputation in lower extremity cases.18 The prevalence of osteomyelitis in full-thickness pressure ulcers ranges from 17%19 to 32%18 and in a prospective histologic and pathologic studies20 of bone cultures, the average cost of treatment was found to be $59,600. Despite the high prevalence of osteomyelitis, no large randomized controlled studies have been conducted with antibiotics nor have specific guidelines been promulgated.
Pathogenesis of Pressure Ulcers
Many studies have tried to explain the etiological pathways involved in the development of pressure ulcers, although precise understanding remains unclear. Similarly, little is known about the pathogenesis of pressure ulcers. Immobilized, bed-bound, elderly patients are the most likely to develop these ulcers. 21 One postulated contributing factor is that ischemia occurs when static stress on the tissue is greater than the pressure in capillaries, restricting the blood flow to the area.22 It has been shown that aged rabbits with chronically ischemic ear wounds fail to heal, suggesting that wound healing impairment is additive, if not synergistic, when age and ischemia are combined. 23 However, the skin break occurs in the epidermis, which is not vascularized, indicating that pressure has multiple pathogenic effects in addition to local ischemia. The initial break occurs in the epidermis and the process progresses toward the deep tissue. However, other studies using animal models have demonstrated damage to the deeper tissues such as muscle before changes in skin occur.24 This also raises the question of how compression (pressure) affects mechanical properties of the skin and epidermis in particular, leading to this breach. Recent in vitro studies25,26 have shown that the deformation of cells within tissues following sustained compression could be an important inducer of cell damage. Other in vivo studies27 have shown that large tissue deformation, in conjunction with ischemia, provide the main trigger for irreversible muscle damage.
Other factors such as age and diabetes also may play a role; however, wounds in elderly patients can be expected to heal, if at a slower rate.28 Recent data from a study29 comparing young db/db mice, aged db/db mice, age-matched non-db/db control, and wild type mice suggest it is the combination of age and diabetes that has been shown to have the most detrimental impact on a wound’s mechanical properties, collagen deposition, and epithelialization.
Pressure ulcers do not naturally occur in animals; therefore, establishing a true animal model has been difficult. These restrictions resulted in limited knowledge of the etiology and pathogenesis of pressure ulcers; the knowledge gained thus far comes only from analyses of human tissues, limited observations, biopsies, and wound fluid analyses.30-37 Research endeavors found elevated levels of IL1; TNF-α; MMP-1, 2, 3, 9, 10, 13; degradation of tenascin-c and fibronectin; and increased pro-collagen expression. However, the possible implications of mechanical load (pressure, static compressive stress) on cellular and molecular processes are yet to be elucidated because Stage I pressure ulcer biopsies from patients are impossible to obtain.
Once formed, pressure ulcers are chronic wounds with physiologic impairments to the wound healing process.6-8 Normal wounding activates keratinocyte migration and proliferation, paralleled by changes in cellular adhesion and cytoskeletal content that promote healing.8 In chronic wounds, keratinocyte migration is inhibited and growth and differentiation are deregulated by the Wnt signaling pathway.8 Specifically, activation of the protein β-catenin (as evidenced by its nuclear presence) and induced expression of the oncogene c-myc lead to inhibition of keratinocyte migration, resulting in a chronic wound that is susceptible to infection. In summary, the development of severe pressure ulcers is generally considered to be multifactorial in nature.
Pressure Ulcer Stages
Pressure ulcers most often occur in the trochanteric, ischial, heel, and sacral areas.38 Susceptibility increases with external (pressure, friction, shear force, and moisture) and internal factors (age, malnutrition, anemia, nervous regulation of local blood flow, and diabetes).39-44 The National Pressure Ulcer Advisory Panel45 has classified four Stages of ulcers, with Stage IV ulcers at risk for underlying osteomyelitis. Multiple scales have been developed to identify high-risk patients13,46-48; however, these scales are variable and clinical judgment was found to be the most reliable indicator of risk.49,50 Early diagnosis and proper treatment of lower stage ulcers are crucial to prevent wound progression. Misdiagnosis and inadequate or improper treatment can lead to progression of the pressure ulcer and consequently increase the risk of complications such as osteomyelitis, sepsis, and amputation.51-53
Osteomyelitis: Pathogenesis, Clinical Presentation, and Complications
In Stage IV pressure ulcers, causative micro-organisms or bacteria can migrate to nearby bone, causing osteomyelitis. Common pathogens in pressure ulcer-associated osteomyelitis include Staphylococcus aureus, Enterobacteriaceae, Streptococcus, Pseudomonas species, and anaerobic bacteria.17-19 Following infection, leukocytes release enzymes that combine with other inflammatory factors to promote tissue necrosis and the destruction of bone cells.54,55 The inflammatory process spreads into the bone’s blood vessels, impairing flow; the resulting ischemia creates areas of devitalized, infected bone. Delivery of certain antibiotics is impaired to this avascular area,56-58 limiting efficacy. During infection, growth factors and cytokines associated with osteoclast and osteoblast activity are also present in abnormal local concentrations, affecting bone structure.59-61 These changes are not well characterized in osteomyelitis.
In general, osteomyelitis can manifest as a poorly healing wound with or without systemic effects, including fever, leukocytosis, and sepsis.17,18,51,62 The infection can involve bone marrow, cortex, and periosteum as well as surrounding soft tissue and may result from a contiguous source of infection.63,64 Older patients have an increased risk due to a higher general incidence of diseases and conditions (ie, pressure ulcers) and/or surgical procedures that predispose them to osteomyelitis.29,62
Osteomyelitis may be classified in several ways,65,66 most commonly acute or chronic.67,68 Acute osteomyelitis is a first episode that is resolved in fewer than 6 weeks. Chronic osteomyelitis lasts longer than 6 weeks or recurs after initial infection and is characterized by persistence of micro-organisms, low-grade inflammation, the presence of dead bone (sequestrum), and fistulous tracts.69 However, these classification schemes do not affect evaluation or management in osteomyelitis associated with Stage IV pressure ulcers largely because these ulcers are treated the same regardless of osteomyelitis classification.
Bone infection under an ulcer often is present for an indeterminate period before diagnosis; all bone infections resulting from a Stage IV pressure ulcers should be considered chronic. The development of a standardized protocol for the prevention, diagnosis, and therapy of pressure ulcers with underlying osteomyelitis is vital to preventing complications such as sepsis associated with Stage IV pressure ulcers. The purpose of this literature and chart review was to develop and evaluate outcomes of an evidence-based protocol for the treatment of osteomyelitis in Stage IV pressure ulcers.
Methods
Literature review. To identify treatment options and evaluate the evidence for all treatment steps regarding osteomyelitis and pressure ulcers, a Pubmed literature search of English-language articles published from 1980 to 2007 was conducted using the search phrases osteomyelitis pressure ulcer, osteomyelitis, pressure ulcer, osteomyelitis antibiotics, osteomyelitis diagnose, and osteomyelitis radiography. All located articles and their associated reference lists were reviewed for clinical relevance; all levels of evidence were included for articles meeting the search criteria.
Patient chart review using wound electronic medical record (WEMR). The records of patients with osteomyelitis underlying pressure ulcers at a university-based hospital inpatient wound clinic were analyzed to provide a specific treatment protocol. Records review was supervised by the senior author. A trained research fellow and a post-doctoral fellow reviewed the records of all patients treated over 3 years (2004–2007), specifically examining data entered into the WEMR. The WEMR was designed using Microsoft Access® and is a relational database with 137 fields for clinical data entry including a digital photograph of the wound; a real-time wound healing rate graph (length, width, depth, and area as a function of time); wound location (as specific as toe or malleolus); drainage, cellulitis, and/or pain; fever; ambulation status; degree of undermining; current treatment; summary of the patient’s medical history; laboratory data; radiology and pathology reports; wound culture reports; medications; and wound treatments. The database output function displays a single data sheet and can be viewed online or printed. This data sheet contains digital photographs of the wound, a graph that trends wound area and depth, and the most recent laboratory data with color-coding for abnormal results.
The WEMR database was culled to identify pressure ulcer patients diagnosed with osteomyelitis, as defined by a positive bone scan or MRI and/or pathology reports showing bone necrosis or acute inflammation. Although the evidence-based guidelines presented herein were not formalized in the authors’ hospital before publication of this manuscript, previous treatment of all patients with osteomyelitis underlying a pressure ulcer was based on, and in accordance with, the same principles. Unexpected returns to the OR, complications, deaths before discharge, and condition upon discharge were recorded for all patients undergoing bone debridement. Data from patients with at least two WEMR records (and corresponding wound areas) following final bone debridement were further analyzed to determine healing trends. Descriptive statistics were calculated using Microsoft Excel (Microsoft Co., Redmond, WA). This research was approved by the Institutional Review Board of Columbia University (IRB–AAAB4487).
Results
More than 300 articles of various levels of evidence and study type (eg, randomized trials, prospective studies, and retrospective studies) were found. The following protocol was developed based on a review of the literature on treatment options for osteomyelitis as well as a retrospective chart review of outcomes (using the WEMR) for patients treated for osteomyelitis. It must be emphasized that this protocol to treat osteomyelitis in Stage IV pressure ulcers must be initiated with the patient (or the guardian) and the primary medical physician and bedside nurse. The protocol consists of the following:
1. Acknowledge that every patient with Stage IV pressure ulcer is at risk for developing osteomyelitis.
2. Clinically evaluate for local or systemic signs of infection upon initial presentation.
3. Radiographically evaluate (magnetic resonance imaging [MRI] or bone scan).
4. Surgically debride all nonviable tissue and/or scarred and infected bone (may be repeated).
5. Obtain pathologic reports from sterile bone biopsy and support findings with deep microbial cultures.
6. Provide systemic antimicrobial therapy based on the micro-organisms identified.
7. Perform tissue reconstruction following resolution of infection.
1. Acknowledge that every patient with Stage IV pressure ulcer is at risk for developing osteomyelitis. Early recognition and intervention are vital to successful treatment of osteomyelitis in Stage IV pressure ulcers. Diagnosis is often difficult due to variability in symptom manifestation, overlying soft-tissue inflammation, and reactive bone formation. Clinical studies have shown that delayed wound healing may be the only clinical sign of osteomyelitis.52,70 Every patient must have pressure ulcer areas examined daily.71 Together, the primary care physician and the wound care specialist must establish a plan and initiate the treatment protocol once osteomyelitis is suspected. Studies72 of the efficacy of traditional diagnostic methods in the presence of overlying ulceration have been shown to have variable results because of inconsistent methodology. Therefore, an accurate diagnosis may require a combination of diagnostic approaches.
2. Clinically evaluate the patient for local or systemic signs of infection upon initial presentation. Although neither sensitive nor specific enough for definitive diagnosis, clinical evaluation is a vital first step in identifying osteomyelitis complications in pressure ulcers. Typical signs of soft tissue infection such as warmth, erythema, local tenderness, purulent discharge, odor, and exposed bone should raise suspicion for osteomyelitis and prompt further study, but these signs alone do not confirm the diagnosis.18,19 In studies19 assessing the accuracy of clinical evaluation of osteomyelitis, when patients clinically evaluated for osteomyelitis subsequently underwent percutaneous needle bone biopsy, the sensitivity and specificity of clinical evaluation in diagnosing osteomyelitis has been shown to be between 33% and 60%.
Laboratory values. Laboratory data collected during the initial evaluation should include complete blood count with manual differential, prothrombin time, partial thromboplastin time, basic metabolic panel, lipid profile, hemoglobin A1c level, hepatic function panel, pre-albumin level, erythrocyte sedimentation rate (ESR), thyroid stimulating hormone level, and urinary micro-albumin level. These tests are important to establish baseline values and should be routinely measured during the treatment of chronic wounds because they reflect the status of comorbid medical conditions such as diabetes and hypercholesterolemia as well as nutrition status.73 Elevated levels of C-reactive protein, increased ESR, leukocytosis, and positive blood cultures are common signs of infection, but have low specificity for osteomyelitis. 17,74-77 These tests are not specific enough to diagnose osteomyelitis; abnormal values should only be used to raise clinical suspicion and evaluate treatment.
3. Radiographically evaluate (MRI or bone scan).
X-rays. Although x-rays are commonly used in evaluating osteomyelitis in patients with Stage IV pressure ulcers, the literature suggests that the role of x-rays for diagnosis is limited. Plain films show soft tissue swelling, narrowing or widening of joint spaces, bone destruction, periosteal and endosteal reactive changes, heterotopic new bone formation, sclerosis, and cortical thickening and irregularities.78-80 The bony changes associated with osteomyelitis, such as periosteal reactive changes or erosion, also may manifest in noninfected bone.62,79 Although it is important to interpret old and new radiographs together to accurately assess change, a literature review conducted by Wrobel et al72 concluded that in the presence of an overlying ulcer, the diagnostic sensitivity and specificity of plain films are approximately 50% and 80%, respectively. Therefore the main role of plain-film radiography is to support clinical suspicion of infection, to diagnose severe osteomyelitis, or to guide further diagnostic methods and/or bone biopsy.81
CT scan. Prospective studies have shown that high-resolution techniques such as computed tomography (CT) and MRI may reveal bone destruction or alteration even when plain radiographs are normal.78-80 CT is capable of viewing cortical bone and identifying dead bone, yet its sensitivity has been found to be only 11% for diagnosing osteomyelitis underlying pressure ulcers in a prospective trial of 61 spinal cord-injured patents whose CT scan reports were compared to pathology reports.70 Nevertheless, it may be most useful in assessing the extent of soft tissue damage.85,86
MRI. MRI is capable of viewing both bone and soft tissue changes — it is the test of choice because it provides an anatomical guide for adjacent bone disease and soft tissue involvement. In a prospective study of 62 patients with diabetes to detect the presence and extent of osteomyelitis, biopsy and clinical follow-up were used to establish the diagnosis; the primary MRI characteristics of osteomyelitis result from marrow edema and infiltration of inflammatory cells.87,88 The two most consistent MRI characteristics of osteomyelitis involve abnormal bone marrow signals.89 MRI is particularly valuable for early diagnosis because it reveals initial bone marrow edema.90 In their retrospective study of 59 patients with pressure ulcers, Huang et al91 showed that MRI had a sensitivity and specificity of 98% and 89%, respectively, in detecting osteomyelitis, meaning a negative MRI result nearly rules out osteomyelitis.72
A commonly perceived drawback of MRI is its high cost, yet it is cost-effective compared to other diagnostic modalities such as combination three-phase bone scan (TPBS) and indium-111-labeled white blood cell (WBC) or gallium-67 citrate scanning.88
Indium scans. Indium (111)-labeled WBC scans use radiolabeled leukocytes that accumulate in sites of inflammation and infection and in the bone marrow. The scans have a reported sensitivity and specificity of 100% and 50%, respectively, after studying 11 patients with Stage IV pressure ulcers.92 High sensitivity and low specificity make this technique best suited to rule out osteomyelitis. The high costs and variable specificity of radionuclide scintigraphy for osteomyelitis limits its use as a primary diagnostic test.92
4. Surgically debride all nonviable tissue and/or scarred and infected bone. Current guidelines for the treatment of osteomyelitis in Stage IV pressure ulcers include bone and ulcer debridements, specific antimicrobial therapy, and a comprehensive regimen that includes daily assessment of the skin, mechanical debridement of nonviable tissue, establishing a moist wound healing environment, nutritional supplementation, pressure relief for the wound, elimination of drainage and cellulitis, physical therapy, and palliative care.71,93 Patients with limb ulcers also should be screened for peripheral arterial disease using noninvasive flow studies (eg, ankle brachial index <0.9 and/or suppressed waveforms in pulse-volume recordings [PVRs]) and revascularization should be considered in patients with peripheral arterial disease.28,73
Surgical debridement of nonviable tissue and/or scarred and infected bone is the foundation for treatment of osteomyelitis underlying Stage IV pressure ulcers.94-96 Antibiotics alone are not adequate because studies have shown that dead bone and surrounding infected areas have minimal vascular supply, which may result in decreased local antibiotic levels.56,58, 95,97,98 Resected bone should be sent for pathology and culture and samples from the remaining bone also should be obtained. If the deepest bone sample is positive for the presence of bacteria or has evidence of necrosis or scar tissue by pathology, further sharp debridement is indicated.95 Debridement should be continued until deep bone cultures are negative95 and the wound is decreasing in area. Expert opinion suggests that bony prominences underlying the ulcer, even if healthy, should be removed to prevent potential ulceration points in the permanently bed-bound patient.99 An area of undermining often may be found over the bone; in such cases, the soft tissue including muscle may have to be debrided. Most Stage IV pressure ulcers heal from the base up; in the authors’ experience, removal of infected tissue below undermining will expose the entire wound base and promote more rapid healing.
5. Obtain pathologic reports from sterile bone biopsy and support with deep microbial cultures. Pathologic examination of the bone is the most accurate way to diagnose osteomyelitis associated with pressure ulcers. Regardless of radiological diagnosis, a bone biopsy should be obtained when osteomyelitis is suspected. A positive diagnosis is based on evidence of necrosis or acute inflammatory cells, including polymorphonuclear leukocytes (PMN), aggregates of lymphocytes, and/or plasma cells and bacteria.17-19,99,100 Pressure-related changes are usually characterized by reactive bone formation or fibrosis without inflammatory changes.18 If possible, multiple bone samples should be obtained from different locations to increase diagnostic accuracy.18 A prospective trial of 61 pressure ulcers by Lewis et al70,76 showed that bone biopsy sensitivity ranges from 73% to 87% and specificity ranges from 93% and 96%. Although no studies have been conducted on the diagnostic sensitivity and specificity of bone samples taken during wound debridement, pathology results of aseptically obtained samples are considered the gold standard.18,101
The diagnostic value of bone cultures for pressure ulcer-related osteomyelitis is controversial due to problems differentiating between bone and adjacent soft tissue infection.16,18,19 There is a general consensus that bone cultures should be used to guide antimicrobial therapy because they may identify the responsible organisms not present in superficial or deep cultures.18,102,103
6. Provide systemic antimicrobial therapy based on the micro-organisms identified. Systemic broad-spectrum antibiotics should be given immediately after bone debridement for osteomyelitis underlying pressure ulcers. Patients initially treated with intravenous antibiotics may be switched to oral medications when appropriate. Antibiotic selection should be based on cultures obtained from bone biopsy, although initial treatment with broad-spectrum antibiotics may be indicated while culture results are pending. Common intravenous treatment regimens based on microbial susceptibility include linezolid,104 vancomycin,105,106 or daptomycin107 for methicillin-resistant S. aureus (MRSA), and piperacillin-tazobactam,108 ticarcillin,109 cefepime or ceftazidime110 for Pseudomonas organisms. Anaerobes are susceptible to clindamycin.111 The drug of choice in the absence of MRSA or Pseudomonas is ertapenem because it is broad-spectrum, convenient (once-a-day dosing), and cost-effective compared to piperacillin-tazobactam; evidence from a randomized controlled trial112 demonstrated efficacy in other types of wounds (ie, diabetic foot ulcers). Due to variable efficacies and the prevalence of resistant organisms, antibiotic selection may require consultation with an infectious disease specialist.
7. Perform tissue reconstruction following resolution of infection. Debridement of bone and tissue may leave dead space that requires significant growth or a myocutaneous flap following the resolution of bone infection. One option is bone grafting, which utilizes cancellous bone capable of revascularization to replace osseous loss.113,114 The modified Papineau technique utilizes open cancellous bone grafting on a granulated tissue base with vacuum-assisted closure (V.A.C.®; KCI, San Antonio, TX).114 Prospective studies115,116 in patients with fractures have shown that the recurrence rate of osteomyelitis following cancellous bone grafts is between 4% and 9%.
Local myocutaneous flaps also can be used for ulcer coverage. The success rate for a myocutaneous flap following pressure ulcer debridement has been found to be as high as 90% in eligible patients when used in conjunction with proper antibiotic coverage.117 Myocutaneous flaps have the advantage of providing vascular supply to both muscle and skin and have been shown to be superior to skin flaps in treating pressure ulcers and containing infection.118 One retrospective study119 of 114 patients reported an 83% healing rate for the treatment of chronic pressure ulcers with myocutaneous flaps, with 14% exhibiting wound edge dehiscence, 9% showing partial flap necrosis, and an overall complication rate of 37%. Fasciocutaneous rotational flaps have been reported to have similar outcomes but are less durable.120 Experimentation with osteocutaneous flaps to the tibia has shown promise121,122 but future studies are needed to determine their relevance to pressure ulcers. Myocutaneous flaps are currently preferred for covering deep pressure ulcers. 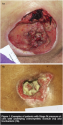
Standard Pressure Ulcer Protocol
Standard protocol for treatment of pressure ulcers should be followed throughout the treatment of underlying osteomyelitis. Protocols include using dressings to create a moist wound healing environment, nutritional supplementation, pressure relief, objective wound measurements, elimination of drainage and cellulitis, mechanical debridement of all nonviable tissue, and physical therapy.71 Topical moist wound healing may be provided using cadexomer iodine,123-125 collagenase,126,127 or silver sulfadiazine.128 Optional biological therapies include growth factors — ie, platelet-derived growth factor (PDGF)-BB,129-131 acellular matrices132 and a bilayer of human living keratinocytes and fibroblasts (also known as human skin equivalent).133 According to animal and in vitro studies,134-138 multiple compounds that stimulate bone regeneration remain the promising therapeutic approach to healing these wounds, yet none to date have been tested for bone regeneration and healing.
Protocol of Care Outcomes
Using the WEMR, charts from 177 consecutive patients with at least one pressure ulcer were reviewed for the presence of osteomyelitis. Of those, 50 had osteomyelitis in 62 out of 233 pressure ulcers. The estimated prevalence of osteomyelitis based on pathology in patients with pelvic pressure ulcers at the time of initial debridement from this review was 33%. Nine patients with 13 pressure ulcers were not debrided to bone and therefore were excluded from the outcome analysis. The remaining 41 patients with 49 pressure ulcers complicated by osteomyelitis underwent 87 bone debridements (see Table  1). Patients initially were encountered as inpatients (as they were receiving operative debridement) and were subsequently seen in the outpatient clinic for follow-up. The WEMR was used to track wound area and other variables regardless of the setting where the patient was seen. The average time to discharge following bone debridement was 4.3 ± 5.7 days. The average follow up time after final bone debridement was 20.6 ± 26.7 weeks. Eight patients experienced complications associated with the treatment of osteomyelitis: two developed Clostridium difficile infection, two experienced postoperative hypotension, one experienced postoperative anemia (requiring transfusion), one patient was discharged to palliative care with unresolved fever spikes, one returned to the OR for bleeding, and one patient had a below-knee amputation (BKA) approximately 2 months after the last heel bone debridement. For all admissions, no deaths occurred prior to discharge.
1). Patients initially were encountered as inpatients (as they were receiving operative debridement) and were subsequently seen in the outpatient clinic for follow-up. The WEMR was used to track wound area and other variables regardless of the setting where the patient was seen. The average time to discharge following bone debridement was 4.3 ± 5.7 days. The average follow up time after final bone debridement was 20.6 ± 26.7 weeks. Eight patients experienced complications associated with the treatment of osteomyelitis: two developed Clostridium difficile infection, two experienced postoperative hypotension, one experienced postoperative anemia (requiring transfusion), one patient was discharged to palliative care with unresolved fever spikes, one returned to the OR for bleeding, and one patient had a below-knee amputation (BKA) approximately 2 months after the last heel bone debridement. For all admissions, no deaths occurred prior to discharge.
Twenty (20) patients with 21 pressure ulcers had at least two WEMR records 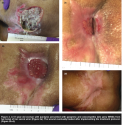 following final bone debridement (see Table 2). Digital photographs (see Figure 1) show an example of a patient with an ischial ulcer and a trochanteric ulcer. Sixteen wounds (76%) had decreased in area at the time of their final visit following final bone debridement. As shown in Figure 2, one patient who presented with gangrene of her Stage IV pressure ulcer, completely re-epithelialized, though another patient in this same group had a BKA. Another patient in this group also had a severe pressure ulcer but under treatment protocol improved significantly (see Figure 3). Five wounds increased in area following final bone debridement.
following final bone debridement (see Table 2). Digital photographs (see Figure 1) show an example of a patient with an ischial ulcer and a trochanteric ulcer. Sixteen wounds (76%) had decreased in area at the time of their final visit following final bone debridement. As shown in Figure 2, one patient who presented with gangrene of her Stage IV pressure ulcer, completely re-epithelialized, though another patient in this same group had a BKA. Another patient in this group also had a severe pressure ulcer but under treatment protocol improved significantly (see Figure 3). Five wounds increased in area following final bone debridement.
Discussion
Osteomyelitis of Stage IV pressure ulcers should be treated with systemic antibiotics and surgical debridement followed by topical therapy. Serial debridements are often necessary to remove all the infected bone. Despite the inherent limitations of retrospective chart reviews, results of the authors’ data confirm the safety of this procedure and that wound area may begin to decrease once bone infection is resolved.18,19
In one clinical study14 of a VA population, the 6-month mortality rate for patients with Stage IV ulcers was been reported to be as high as is 68.9%. In this study, no operative mortalities were reported during an average follow-up of 21 weeks, suggesting that the procedure is safe, although further longitudinal study is needed to determine whether a surgical treatment protocol of osteomyelitis may improve long-term mortality. If the patient is eligible, myocutaneous flaps are recommended.117-119 However, it is important to note that many candidates for myocutaneous flap surgery have an unacceptable complication risk — their other morbidities may adversely affect healing and/or necessitate invasive procedures or transfers that preclude them from remaining bed-bound for a month.
Preventing Stage IV pressure ulcers will reduce the prevalence of osteomyelitis. Educating patients, their families, and clinicians on the severity of the disease, risk factors, and treatment is critical to minimizing associated morbidity and mortality. It has been shown in multiple prospective studies139-141 that moderate reductions in pressure ulcer prevalence can be been achieved by limiting the external risk factors of pressure, friction, moisture, and shear force. An alternative approach focuses on the early diagnosis and proper treatment of lower Stage pressure ulcers, which may halt ulcer progression to deeper tissue and/or bone.71
The WEMR is an effective tool for the documentation of clinical data relevant to care of the patient with severe pressure ulcers and provides a convenient platform to follow outcomes such as wound area. Because most studies of patients with pressure ulcers evaluate a particular treatment and therefore usually exclude osteomyelitis, few reports exist in the literature pertaining to healing 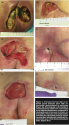 outcomes in patients with osteomyelitis. This study suggests that by implementing an evidence-based approach, most patients’ wounds can ultimately decrease in area, although extended treatment may be necessary. This study was limited because of its retrospective design but the findings highlight the need for a prospective study evaluating the efficacy of the described protocol for treatment of osteomyelitis.
outcomes in patients with osteomyelitis. This study suggests that by implementing an evidence-based approach, most patients’ wounds can ultimately decrease in area, although extended treatment may be necessary. This study was limited because of its retrospective design but the findings highlight the need for a prospective study evaluating the efficacy of the described protocol for treatment of osteomyelitis.
The majority of patients with osteomyelitis in a Stage IV pressure ulcer present from a long-term healthcare setting after development of sepsis. In this study, 76% of the wounds that had been seen for more than two consecutive visits and documented in the WEMR demonstrated a decrease in area. Thus, the expectation is that with a daily objective examination and appropriate treatment, nearly all pressure ulcers can improve. Emphasis must be placed on working with the patient, family, and caregivers to ensure weekly follow-up when treating osteomyelitis. With an electronic medical record such as the WEMR, the clinician can make objective treatment decisions.
Conclusion
Based on a review of the literature, a seven-step protocol for treatment of osteomyelitis in pressure ulcers was developed. Bed-bound and/or critically ill patients require daily skin inspections. An electronic documentation system, which includes a photograph of the skin, such as the one described in this study, may alert clinicians to worsening pressure ulcers, allowing intervention before progression to Stage IV. When osteomyelitis is diagnosed, utilization of the aforementioned treatment protocol has been found to be safe and supported by current literature. The majority of Stage IV pressure ulcers can decrease in area, even with osteomyelitis, under treatment protocol implemented with a WEMR.
Acknowledgments
The authors thank all members of the Wound Healing Program, especially Jeannine Lewis and Fay-Marie Hall, for their thoughtful review and suggestions on this project. Mr. Rennert is a medical student, The Ohio State University School of Medicine, Columbus, OH. Dr. Golinko is a surgical resident and previous post-doctoral fellow and Dr. Yan is a fellow, Division of Wound Healing and Regenerative Medicine, Department of Surgery, New York University School of Medicine, New York, NY. Dr. Flattau is an Assistant Professor, Montefiore Medical Center, Department of Social and Family Medicine, Bronx, NY. Dr. Tomic-Canic is Professor of Dermatology and Cutaneous Surgery, Miller School of Medicine, University of Miami, Miami, FL. Dr. Brem is Associate Professor of Surgery and Division Chief, Division of Wound Healing and Regenerative Medicine, Helen & Martin Kimmel Wound Center, Department of Surgery, New York University School of Medicine. Please address correspondence to: Harold Brem, MD, FACS, Helen & Martin Kimmel Wound Center, Department of Surgery, New York University School of Medicine, 301 East 17th Street, Room 1027, New York, NY 10003; email: Harold.brem@nyumc.org.
1. Lyder CH. Pressure ulcer prevention and management. JAMA. 2003;289(2):223–226.
2. Hospitalizations related to pressure sores. 2003. Available at: www.hcup–us.ahrq.gov/reports/statbriefs/sb3.pdf. Accessed February 1, 2007.
3. Groeneveld A, Anderson M, Allen S, et al. The prevalence of pressure ulcers in a tertiary care pediatric and adult hospital. J WOCN. 2004;31(3):108–120.
4. Davis CM, Caseby NG. Prevalence and incidence studies of pressure ulcers in two long–term care facilities in Canada. Ostomy Wound Manage. 2001;47(11):28–34.
5. Garber SL, Rintala DH. Pressure ulcers in veterans with spinal cord injury: a retrospective study. J Rehabil Res Dev. 2003;40(5):433–41.
6. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–493.
7. Mostow EN. Diagnosis and classification of chronic wounds. Clin Dermatol. 1994;12(1):3–9.
8. Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167(1):59–69.
9. Allman RM, Goode PS, Burst N, Bartolucci AA, Thomas DR. Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Adv Wound Care. 1999;12(1):22–30.
10. Landi F, Onder G, Russo A, Bernabei R. Pressure ulcer and mortality in frail elderly people living in community. Arch Gerontol Geriatr. 2007;44(suppl 1):217–223.
11. Redelings MD, Lee NE, Sorvillo F. Pressure ulcers: more lethal than we thought? Adv Skin Wound Care. 2005;18(7):367–372.
12. Schryvers OI, Stranc MF, Nance PW. Surgical treatment of pressure ulcers: 20–year experience. Arch Phys Med Rehabil. 2000;81(12):1556–1562.
13. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for Predicting Pressure Sore Risk. Nurs Res. 1987;36(4):205–210.
14. Brown G. Long–term outcomes of full–thickness pressure ulcers: healing and mortality. Ostomy Wound Manage. 2003;49(10):42–50.
15. Ford CN, Reinhard ER, Yeh D, et al. Interim analysis of a prospective, randomized trial of vacuum–assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg. 2002;49(1):55–61.
16. Han H, Lewis VL, Jr., Wiedrich TA, Patel PK. The value of Jamshidi core needle bone biopsy in predicting postoperative osteomyelitis in grade IV pressure ulcer patients. Plast Reconstr Surg. 2002;110(1):118–122.
17. Thornhill-Joynes M, Gonzales F, Stewart CA, et al. Osteomyelitis associated with pressure ulcers. Arch Phys Med Rehabil. 1986;67(5):314–318.
18. Sugarman B, Hawes S, Musher DM, Klima M, Young EJ, Pircher F. Osteomyelitis beneath pressure sores. Arch Intern Med. 1983;143(4):683–688.
19. Darouiche RO, Landon GC, Klima M, Musher DM, Markowski J. Osteomyelitis associated with pressure sores. Arch Intern Med. 1994;154(7):753–758.
20. Hirshberg J, Rees RS, Marchant B, Dean S. Osteomyelitis related to pressure ulcers: the cost of neglect. Adv Skin Wound Care. 2000;13(1):25–29.
21. Brandeis GH, Morris JN, Nash DJ, Lipsitz LA. The epidemiology and natural history of pressure ulcers in elderly nursing home residents. JAMA. 1990;264(22);2905–2909.
22. Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005;23(4):403–412.
23. Bonomo SR, Davidson JD, Tyrone JW, Lin X, Mustoe TA. Enhancement of wound healing by hyperbaric oxygen and transforming growth factor beta3 in a new chronic wound model in aged rabbits. Arch Surg. 2000;135(10):1148–1153.
24. Salcido R, Donofrio JC, Fisher SB, et al. Histopathology of pressure ulcers as a result of sequential computer-controlled pressure sessions in a fuzzy rat model. Adv Wound Care. 1994;7(5):23–48.
25. Bouten CV, Breuls RG, Peeters EA, Oomens CW, Baaijens FP. In vitro models to study compressive strain-induced muscle cell damage. Biorheology. 2003;40(1-3):383–388.
26. Breuls RG, Bouten CV, Oomens CW, Bader DL, Baaijens FP. Compression induced cell damage in engineered muscle tissue: an in vitro model to study pressure ulcer aetiology. Ann Biomed Eng. 2003;31(11):1357–1364.
27. Stekelenburg A, Strijkers GJ, Parusel H, Bader D, Nicolay K, Oomens C. The role of ischemia and deformation in the onset of compression–induced deep tissue injury: MRI–based studies in a rat model. J Appl Physiol. 2007;102(5):2002–2011.
28. Brem H, Tomic-Canic M, Tarnovskaya A, et al. Healing of elderly patients with diabetic foot ulcers, venous stasis ulcers, and pressure ulcers. Surg Technol Int. 2003;11:161–167.
29. Brem H, Tomic-Canic M, Entero H, et al. The synergism of age and db/dgenotype impairs wound healing. Exp Gerontol. 2007;42(6):523–531.
30. Barone EJ, Yager DR, Pozez AL, et al. Interleukin–1alpha and collagenase activity are elevated in chronic wounds. Plast Reconstr Surg. 1998;102(4):1023–1027; discussion 1028–10299.
31. Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP–2 and MMP-9. J Invest Dermatol. 1993;101(1):64–68.
32. Saarialho–Kere UK. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res. 1998;290(suppl):S47–S54.
33. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10(1):26–37.
34. Latijnhouwers MA, Bergers M, Veenhuis RT, Beekman B, Ankersmit-Ter Horst MF, Schalkwijk J. Tenascin-C degradation in chronic wounds is dependent on serine proteinase activity. Arch Dermatol Res 1998;290(9):490–496.
35. Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol. 1992;98(4):410–416.
36. Wallace HJ, Stacey MC. Levels of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in chronic venous leg ulcers––correlations to healing status. J Invest Dermatol. 1998;110(3):292–296.
37. Tomic-Canic M, Magnus SA, Oscar MA. Epidermal repair and the chronic wound. In: Rovee DT, Maibach HI (eds). The Epidermis in Wound Healing. Florida: CRC Press LLC;2004:25–57.
38. Scott JR, Gibran NS, Engrav LH, Mack CD, Rivara FP. Incidence and characteristics of hospitalized patients with pressure ulcers: State of Washington, 1987 to 2000. Plast Reconstr Surg. 2006;117(2):630–634.
39. van Marum RJ, Meijer JH, Ribbe MW. The relationship between pressure ulcers and skin blood flow response after a local cold provocation. Arch Phys Med Rehabil. 2002;83(1):40–43.
40. Casimiro C, Garc-de-Lorenzo A, Usan L. Prevalence of decubitus ulcer and associated risk factors in an institutionalized Spanish elderly population. Nutrition. 2002;18(5):408–414.
41. Schubert V, Heraud J. The effects of pressure and shear on skin microcirculation in elderly stroke patients lying in supine or semi-recumbent positions. Age Ageing. 1994;23(5):405–410.
42. Bots TC, Apotheker BF. The prevention of heel pressure ulcers using a hydropolymer dressing in surgical patients. J Wound Care. 2004;13(9):375–378.
43. Fisher AR, Wells G, Harrison MB. Factors associated with pressure ulcers in adults in acute care hospitals. Holist Nurs Pract. 2004;18(5):242–253.
44. Theaker C, Mannan M, Ives N, Soni N. Risk factors for pressure sores in the critically ill. Anaesthesia. 2000;55(3):221–224.
45. National Pressure Ulcer Advisory Panel. Pressure ulcer stages revised by NPUAP. Available at: www.npuap.org/pr2.htm. Accessed January 14, 2009.
46. Jackson C. The revised Jackson/Cubbin Pressure Area Risk Calculator. Intensive Crit Care Nurs. 1999;15(3):169–175.
47. Waterlow J. Pressure sores: a risk assessment card. Nurs Times. 1985;81(48):49–55.
48. Norton D. Calculating the risk: reflections on the Norton Scale. Decubitus. 1989;2(3):24–31.
49. Gould D, Goldstone L, Kelly D, Gammon J. Examining the validity of pressure ulcer risk assessment scales: a replication study. Int J Nurs Stud. 2004;41(3):331–339.
50. Jun Seongsook RN, Jeong Ihnsook RN, Lee Younghee RN. Validity of pressure ulcer risk assessment scales; Cubbin and Jackson, Braden, and Douglas scale. Int J Nurs Stud. 2004;41(2):199–204.
51. Galpin JE, Chow AW, Bayer AS, Guze LB. Sepsis associated with decubitus ulcers. Am J Med. 1976;61(3):346–350.
52. Dimant J, Tanael L. Decubitus ulcers: when to suspect osteomyelitis. Geriatrics. 1987;42(6):74–83.
53. Montgomerie JZ, Chan E, Gilmore DS, Canawati HN, Sapico FL. Low mortality among patients with spinal cord injury and bacteremia. Rev Infect Dis. 1991;13(5):867–871.
54. Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004;76(1):135–144.
55. Yu JJ, Ruddy MJ, Wong GC, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor–dependent signals. Blood. 2007;109(9):3794–3802.
56. Frost HM, Villaneuva AR, Roth H. Pyogenic osteomyelitis: diffusion in live and dead bone with particular reference to the tetracycline antibiotics. Henry Ford Hosp Med Bull. 1960;8:255–262.
57. Fong IW, Rittenhouse BR, Simbul M, Vandenbroucke AC. Bone penetration of enoxacin in patients with and without osteomyelitis. Antimicrob Agents Chemother. 1988;32(6(:834–837.
58. Graziani AL, Lawson LA, Gibson GA, Steinberg MA, MacGregor RR. Vancomycin concentrations in infected and noninfected human bone. Antimicrob Agents Chemother. 1988;32(9):1320–1322.
59. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–137.
60. Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–311.
61. Klosterhalfen B, Peters KM, Tons C, Hauptmann S, Klein CL, Kirkpatrick CJ. Local and systemic inflammatory mediator release in patients with acute and chronic posttraumatic osteomyelitis. J Trauma. 1996;40(3):372–378.
62. Livesley N, Chow A. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002:35(11):1390–1396.
63. Cohen MD, Cory DA, Kleiman M, Smith JA, Broderick NJ. Magnetic resonance differentiation of acute and chronic osteomyelitis in children. Clin Radiol. 1990;41(1):53–56.
64. Gilbert DN, Tice AD, Marsh PK, Craven PC, Preheim LC. Oral ciprofloxacin therapy for chronic contiguous osteomyelitis caused by aerobic gram–negative bacilli. Am J Med. 1987;82(4A):254–258.
65. Weiland AJ, Moore JR, Daniel RK. The efficacy of free tissue transfer in the treatment of osteomyelitis. J Bone Joint Surg Am 1984;66(2):181–193.
66. May JW, Jr., Jupiter JB, Weiland AJ, Byrd HS. Clinical classification of post–traumatic tibial osteomyelitis. J Bone Joint Surg Am. 1989;71(9):1422–1428.
67. Bachur R, Pagon Z. Success of short-course parenteral antibiotic therapy for acute osteomyelitis of childhood. Clin Pediatr. (Phila) 2007;46(1):30–35.
68. Lu WJ, Li B, Bao NR, et al. Treatment of chronic osteomyelitis with one–stage allograft. Chin J Traumatol. 2006;9(5):272–275.
69. Cunha BA. Osteomyelitis in elderly patients. Clin Infect Dis. 2002;35(3):287–293.
70. Lewis VL Jr, Bailey MH, Pulawski G, Kind G, Bashioum RW, Hendrix RW. The diagnosis of osteomyelitis in patients with pressure sores. Plast Reconstr Surg. 1988;81(2):229–232.
71. Brem H, Lyder C. Protocol for the successful treatment of pressure ulcers. Am J Surg. 2004;188(1A suppl):9–17.
72. Wrobel JS, Connolly JE. Making the diagnosis of osteomyelitis. The role of prevalence. J Am Podiatr Med Assoc. 1998;88(7):337–343.
73. Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg. 2006;117:193(7 suppl):193S–209S; discussion 210S–211S.
74. Unkila–Kallio L, Kallio MJ, Eskola J, Peltola H. Serum C–reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics. 1994;93(1):59–62.
75. Floyed RL, Steele RW. Culture–negative osteomyelitis. Pediatr Infect Dis J. 2003;22(8):731–736.
76. Howard CB, Einhorn M, Dagan R, Yagupski P, Porat S. Fine-needle bone biopsy to diagnose osteomyelitis. J Bone Joint Surg Br. 1994;76(2):311–314.
77. Scivoletto G, Fuoco U, Morganti B, Cosentino E, Molinari M. Pressure sores and blood and serum dysmetabolism in spinal cord injury patients. Spinal Cord. 2004;42(8):473–476.
78. Buckland-Wright JC, Lynch JA, Dave B. Early radiographic features in patients with anterior cruciate ligament rupture. Ann Rheum Dis. 2000;59(8):641–646.
79. Tumeh SS, Aliabadi P, Weissman BN, McNeil BJ. Disease activity in osteomyelitis: role of radiography. Radiology. 1987;165(3):781–784.
80. Haas ML, Kennedy AS, Copeland CC, Ames JW, Scarboro M, Slawson RG. Utility of radiation in the prevention of heterotopic ossification following repair of traumatic acetabular fracture. Int J Radiat Oncol Biol Phys. 1999;45(2):461–466.
81. Kaim AH, Gross T, von Schulthess GK. Imaging of chronic posttraumatic osteomyelitis. Eur Radiol. 2002;12(5):1193–1202.
82. Wing VW, Jeffrey RB Jr, Federle MP, Helms CA, Trafton P. Chronic osteomyelitis examined by CT. Radiology. 1985;154(1):171–174.
83. Kapoor A, Page S, Lavalley M, Gale DR, Felson DT. Magnetic resonance imaging for diagnosing foot osteomyelitis: a meta-analysis. Arch Intern Med. 2007;167(2):125–132.
84. Azouz EM. Computed tomography in bone and joint infections. J Can Assoc Radiol. 1981;32(2):102–106.
85. Santiago Restrepo C, Gimenez CR, McCarthy K. Imaging of osteomyelitis and musculoskeletal soft tissue infections: current concepts. Rheum Dis Clin North Am. 2003;29(1):89–109.
86. Ma LD, Frassica FJ, Bluemke DA, Fishman EK. CT and MRI evaluation of musculoskeletal infection. Crit Rev Diagn Imaging. 1997;38(6):535–568.
87. Deely DM, Schweitzer ME. MR imaging of bone marrow disorders. Radiol Clin North Am. 1997;35(1):193–212.
88. Morrison WB, Schweitzer ME, Wapner KL, Hecht PJ, Gannon FH, Behm WR. Osteomyelitis in feet of diabetics: clinical accuracy, surgical utility, and cost-effectiveness of MR imaging. Radiology. 1995;1969(2):557–564.
89. Morrison WB, Schweitzer ME, Batte WG, Radack DP, Russel KM. Osteomyelitis of the foot: relative importance of primary and secondary MR imaging signs. Radiology. 1998;207(3):625–632.
90. Davies AM, Hughes DE, Grimer RJ. Intramedullary and extramedullary fat globules on magnetic resonance imaging as a diagnostic sign for osteomyelitis. Eur Radiol. 2005;15(10):2194–2199.
91. Huang AB, Schweitzer ME, Hume E, Batte WG. Osteomyelitis of the pelvis/hips in paralyzed patients: accuracy and clinical utility of MRI. J Comput Assist Tomogr. 1998;22(3):437–443.
92. Melkun ET, Lewis VL, Jr. Evaluation of (111) indium–labeled autologous leukocyte scintigraphy for the diagnosis of chronic osteomyelitis in patients with grade IV pressure ulcers, as compared with a standard diagnostic protocol. Ann Plast Surg. 2005;54(6):633–636.
93. Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen. 2006;14(6):663–679.
94. Gentry LO, Rodriguez GG. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother. 1990;34(1):40–43.
95. Eckardt JJ, Wirganowicz PZ, Mar T. An aggressive surgical approach to the management of chronic osteomyelitis. Clin Orthop Relat Res. 1994;298:229–239.
96. Greenberg RN, Newman MT, Shariaty S, Pectol RW. Ciprofloxacin, lomefloxacin, or levofloxacin as treatment for chronic osteomyelitis. Antimicrob Agents Chemother. 2000;44(1):164–166.
97. Mathes SJ, Alpert BS, Chang N. Use of the muscle flap in chronic osteomyelitis: experimental and clinical correlation. Plast Reconstr Surg. 1982;69(5):815–829.
98. Clawson DK, Dunn AW. Management of common bacterial infections of bones and joints. J Bone Joint Surg Am. 1967;49(1):164–182.
99. Deloach ED, DiBenedetto RJ, Womble L, Gilley JD. The treatment of osteomyelitis underlying pressure ulcers. Decubitus. 1992;5(6):32–41.
100. Damjanov I, Linder J, Anderson WAD. Anderson's Pathology, 10th ed. St. Louis. MO: Mosby;1996:416–447.
101. Sugarman B. Pressure sores and underlying bone infection. Arch Intern Med. 1987;14793):553–555.
102. Khatri G, Wagner DK, Sohnle PG. Effect of bone biopsy in guiding antimicrobial therapy for osteomyelitis complicating open wounds. Am J Med Sci. 2001;321(6):367–371.
103. Dubey L, Krasinski K, Hernanz–Schulman M. Osteomyelitis secondary to trauma or infected contiguous soft tissue. Pediatr Infect Dis J. 1988;7(1):26–34.
104. Rayner CR, Baddour LM, Birmingham MC, Norden C, Meagher AK, Schentag JJ. Linezolid in the treatment of osteomyelitis: results of compassionate use experience. Infection. 2004;32(1):8–14.
105. Bernard L, Vaudaux P, Vuagnat A, et al. Effect of vancomycin therapy for osteomyelitis on colonization by methicillin-resistant Staphylococcus aureus: lack of emergence of glycopeptide resistance. Infect Control Hosp Epidemiol. 2003;24(9):650–654.
106. Rajaduraipandi K, Mani KR, Panneerselvam K, Mani M, Bhaskar M, Manikandan P. Prevalence and antimicrobial susceptibility pattern of methicillin–resistant Staphylococcus aureus: a multicentre study. Indian J Med Microbiol. 2006;24(1):34–38.
107. Fowler VG, Jr., Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–665.
108. Lodise TP Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended–infusion dosing strategy. Clin Infect Dis. 2007;44(3):357–363.
109. Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F. Systematic review and meta–analysis of antibiotic therapy for bone and joint infections. Lancet Infect Dis. 2001;1(3):175–188.
110. Jones ME, Karlowsky JA, Draghi DC, Thornsberry C, Sahm DF, Nathwani D. Antibiotic susceptibility of bacteria most commonly isolated from bone related infections: the role of cephalosporins in antimicrobial therapy. Int J Antimicrob Agents. 2004;23(3):240–246.
111. Pankuch GA, Jacobs MR, Appelbaum PC. Susceptibilities of 428 gram-positive and -negative anaerobic bacteria to Bay y3118 compared with their susceptibilities to ciprofloxacin, clindamycin, metronidazole, piperacillin, piperacillin-tazobactam, and cefoxitin. Antimicrob Agents Chemother. 1993;37(8):1649–1654.
112. Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded multicentre trial. Lancet. 2005;366(9498):1695–1703.
113. Isaac MR, Kanat IO. Bone grafts in the management of osteomyelitis. J Foot Surg. 1986;25(5):404–406.
114. Archdeacon MT, Messerschmitt P. Modern Papineau technique with vacuum-assisted closure. J Orthop Trauma. 2006;20(2):134–137.
115. Monsivais JJ. Effective management of osteomyelitis after grade III open fractures. J South Orthop Assoc. 1996;5(1):30–36.
116. Tulner SA, Schaap GR, Strackee SD, Besselaar PP, Luitse JS, Marti RK. Long–term results of multiple-stage treatment for posttraumatic osteomyelitis of the tibia. J Trauma. 2004;56(3):633–642.
117. Mathes SJ, Feng LJ, Hunt TK. Coverage of the infected wound. Ann Surg. 1983;198(4):420–429.
118. Bruck JC, Bittemeyer R, Grabosch A, Gruhl L. More arguments in favor of myocutaneous flaps for the treatment of pelvic pressure sores. Ann Plast Surg. 1991;26(1):85–88.
119. Foster RD, Anthony JP, Mathes SJ, Hoffman WY. Ischial pressure sore coverage: a rationale for flap selection. Br J Plast Surg. 1997;50(5):374–379.
120. Wong TC, Ip FK. Comparison of gluteal fasciocutaneous rotational flaps and myocutaneous flaps for the treatment of sacral sores. Int Orthop. 2006;30(1):64–67.
121. Lee KS, Han SB, Baek JR. Free vascularized osteocutaneous fibular graft to the tibia in 51 consecutive cases. J Reconstr Microsurg. 2004;20(4):277–284.
122. Lee KS, Park JW. Free vascularized osteocutaneous fibular graft to the tibia. Microsurgery. 1999;19(3):141–147.
123. Ohtani T, Mizuashi M, Ito Y, Aiba S. Cadexomer as well as cadexomer iodine induces the production of proinflammatory cytokines and vascular endothelial growth factor by human macrophages. Exp Dermatol. 2007;16(4):318–323.
124. Lamme EN, Gustafsson TO, Middelkoop E. Cadexomer-iodine ointment shows stimulation of epidermal regeneration in experimental full-thickness wounds. Arch Dermatol Res. 1998;290(1-2):18–24.
125. Zhou LH, Nahm WK, Badiavas E, Yufit T, Falanga V. Slow release iodine preparation and wound healing: in vitro effects consistent with lack of in vivo toxicity in human chronic wounds. Br J Dermatol. 2002;146(3):365–374.
126. Boxer AM, Gottesman N, Bernstein H, Mandl I. Debridement of dermal ulcers and decubiti with collagenase. Geriatrics. 1969;24(7):75–86.
127. Rao DB, Sane PG, Georgiev EL. Collagenase in the treatment of dermal and decubitus ulcers. J Am Geriatr Soc. 1975;23(1):22–30.
128. Kucan JO, Robson MC, Heggers JP, Ko F. Comparison of silver sulfadiazine, povidone-iodine and physiologic saline in the treatment of chronic pressure ulcers. J Am Geriatr Soc. 1981;29(5):232–235.
129. Landi F, Aloe L, Russo A, et al. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med. 2003;139(8):635–641.
130. Payne WG, Ochs DE, Meltzer DD, et al. Long-term outcome study of growth factor–treated pressure ulcers. Am J Surg. 2001;181(1):81–86.
131. Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen. 1999;7(3):141–147.
132. Muangman P, Engrav LH, Heimbach DM, et al. Complex wound management utilizing an artificial dermal matrix. Ann Plast Surg. 2006;57(2):199–202.
133. Brem H, Balledux J, Bloom T, Kerstein MD, Hollier L. Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: a new paradigm in wound healing. Arch Surg. 2000;135(6):627–634.
134. Komaki H, Tanaka T, Chazono M, Kikuchi T. Repair of segmental bone defects in rabbit tibiae using a complex of beta–tricalcium phosphate, type I collagen, and fibroblast growth factor–2. Biomaterials. 2006;27(29):5118–5126.
135. Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Bone regeneration on a collagen sponge self-assembled peptide-amphiphile nanofiber hybrid scaffold. Tissue Eng. 2007;13(1):11–19.
136. Yamamoto M, Takahashi Y, Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein–2 from a biodegradable hydrogel. Tissue Eng. 2006;12(5):1305–1311.
137. Guizzardi S, Martini D, Bacchelli B, et al. Effects of heat deproteinate bone and polynucleotides on bone regeneration: an experimental study on rat. Micron. 2007;38(7):722–728.
138. Itaka K, Ohba S, Miyata K, et al. Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex nanomicelles. Mol Ther. 2007;15(9):1655–1662.
139. Courtney BA, Ruppman JB, Cooper HM. Save our skin: initiative cuts pressure ulcer incidence in half. Nurs Manage. 2006;37(4):36–40.
140. Hampton S, Collins F. Reducing pressure ulcer incidence in a long-term setting. Br J Nurs. 2005;14(15 suppl):S6–S12.
141. Klay M, Marfyak K. Use of a continence nurse specialist in an extended care facility. Urol Nurs. 2005;25(2):101–108.














