A Cross-sectional Study to Validate Wound Care Algorithms for Use by Registered Nurses
Abstract
Research has shown that providing quality wound care affects outcomes and cost. Heuristic devices that facilitate this process, such as guidelines and algorithms, are widely available, but most have not been validated for use by the practitioners for whom they were designed. Although the validity of one set of wound care algorithms (Solutions®, ConvaTec, Inc., Skillman, NJ) has been established by expert wound clinicians and excellent clinical usage outcomes reported, its validity when used by nonexpert clinicians (eg, staff registered nurses) to expedite the clinical decision-making process has not been established.
Using a cross-sectional, mixed methods, quantitative survey design, a study was conducted to validate use of these algorithms by 204 nonwound expert registered nurses associated with an urban university. Following a very brief explanation, participants (88% women, mean age 34.8 [range 23 to 60] years, 4 to 9 years nursing experience, currently practicing in an acute care setting) were asked to rate 11 components of the algorithms, apply them to a variety of acute and chronic wounds (N = 15), and comment on the research process and algorithm use. The content validity of all components was strong (CVI >0.96); 71% to 98% of nurses selected the correct (primary) or appropriate but not entirely correct (secondary) algorithm; the correct dressing was selected for 75% to 91% of wounds shown. Correct algorithm and dressing selection percents were lower for wounds with necrotic tissue. Intra-rater reliability (two sets of two wounds) was modest but higher for necrotic wounds. A common qualitative theme was insecurity regarding wound assessment. The study results support that the algorithms tested have face and content validity and facilitate the provision of optimal care with a variety of wound types (construct validity) and confirm that wound education improvements for registered nurses are needed.
Potential Conflicts of Interest: Dr. Beitz has received speaker honoraria and research funding and served as a consultant or paid advisory board member; and Ms. van Rijswijk has received research funding and served as a consultant for ConvaTec, Inc., Skillman, NJ. This study was supported by a research grant from ConvaTec, Inc. The study was conducted, the data analyzed, and the manuscript written by the authors who are responsible for the content of this publication. The authors do not have a financial interest in any products discussed in this publication. Solutions® is a Registered Trademark of ConvaTec, Inc., Skillman, NJ.
Please address correspondence to: Janice M. Beitz, PhD, RN, CS, CNOR, CWOCN CRNP, La Salle University, 1900 W. Olney Avenue, Philadelphia, PA 19141; email: beitz@lasalle.edu.
In 1999, Beitz and van Rijswijk1 conducted a content-validation study for the Solutions® Algorithms, a set of eight guidelines designed to help healthcare professionals make evidence and wound assessment-based decisions of care involving wound care expert clinicians. Variables included in the algorithm address factors such as amount of moisture, wound bed status, amount of necrotic tissue, wound depth, and the like. Using a cross-sectional design, 44 registered nurse wound experts completed an 83-item, 4-point Likert-type scale quantifying the degree of validity inherent in the algorithms’ decisions and components and tape-recorded open-ended comments about the content and processes of the algorithms. The wound care algorithms were rated as valid and the content validity index (CVI) for the whole set of algorithms was high (0.86). However, the study identified areas of concern. The algorithms’ study did not assess the ability of these heuristic devices to promote correct wound care selection and results were a reflection only of responses from wound experts (persons formally educated and were board-certified in wound care). A subsequent content validity study2 of a software program based on these algorithms also involved wound care experts.
Because the algorithms were designed to promote optimal wound care (correct assessment and dressing selection) for clinicians who provide most of the care (eg, licensed registered nurses without formal education and certification), the purpose of the present study was to address these important areas by evaluating the content and criterion-related (concurrent) validity of the algorithms among nonwound expert registered nurses. 
Background and Literature Review
By graphically displaying concept interrelationships, algorithms help healthcare professionals’ decision-making and allow users to apply large amounts of information to practical solutions.3,4 By showing the “big picture” or metacognitive perspectives, algorithms help organize thinking, make relationships more meaningful, and highlight crucial decision points.5 Because they greatly affect the quality of patient care, algorithm content and usage need to be research-based. Although many guidelines, clinical pathways, and algorithms have been developed in healthcare (including wound care), to this day few are evidence-based and content-validated for actual end-user utilization.6
Content validity. Content validity is a crucial factor in the use of any instrument and vitally important for maps or algorithms affecting patient safety. Content validity evaluation is a rigorous, usually two-stage process that involves 1) development and 2) judgment quantification.7,8 The development stage of content validation consists of domain (topical area) identification, item generation, and instrument construction. The judgment quantification stage involves rating the degree of item content relevance by experts using an empirical method, similar to the content validity process. Omission of or poor attention to either stage can compromise validity.
The content validity of one general wound care algorithm (Solutions® Algorithms for wound care, ConvaTec, Inc, Skillman, NJ)9 has been established. After face validity was established by a small group of reviewers, 44 expert wound care clinicians participated in the Beitz and van Rijswijk study.1 Because of the original objective of the these algorithms (ie, to aid nonexpert caregivers in the assessment of wounds and selection of appropriate primary dressings), validity depends on the adequacy with which the critical characteristics of wounds healing by secondary intention are sampled.
In the 1999 algorithm validity study,1 the judgment quantification component of content-related validity was obtained from wound experts using the content validity index (CVI) in a process developed by Waltz and Bausell10 and modified by Lynn.8 Experts rated the content relevance of each component or subcomponent of an algorithm or instrument by using a 4-point, Likert-type rating scale. Lynn8 recommended standardizing options to read: 1) not relevant; 2) unable to assess relevance without item revision or item is in need of such revision that it would no longer be relevant; 3) relevant but needs minor attention; 4) very relevant and succinct. For the current study and the earlier Beitz and van Rijswijk study, option 1 was modified (for readability and clarity) to read not relevant/appropriate: option 2, unable to assess relevance without revision; option 3, relevant but needs minor alteration; option 4, very relevant and appropriate. Use of a four-part scale (as opposed to, for example, a 5-point scale) also addressed the possible negative influence of having a scale that includes an ambivalent middle rating.10
In the 1999 study,1 each expert rated the relevance of the component or segment to wound care and the wound care algorithms in total. Other well known wound instruments, such as the Pressure Sore Status Tool (PSST)11 were established in this manner. An issue with the 1999 research study was that the algorithm content was evaluated by expert wound clinicians, not the population for whom the care guides were written. Hence, the current study was proposed. In addition to quantitative analysis of algorithmic components, researchers also were provided insights via qualitative analysis of feedback from participants in response to open-ended questions, analysis of which may generate identifiable themes of strength or concern. This type of information was also a goal in the current study. Finally, because evidence of algorithmic use in promoting optimal patient care is limited,4,12-14 validity of usage was an important goal of this study.
The Solutions® Algorithms
Algorithms structure. The algorithms tested take users through the steps of developing optimal patient and wound interventions based on the basic principles of pressure ulcer prevention, wound care, wound assessment, consideration of expected outcomes, and goals of care.7,15,16 They consist of one pressure ulcer prevention and eight wound care algorithms (see Figure 1). Wound assessment variables included in the algorithms that require monitoring and affect treatment decisions are based on the PSST and include amount of moisture, wound bed status/amount of necrotic tissue, depth, surrounding skin condition, and condition of the wound edges.11,17 Content validity and reliability of the PSST have been established in several studies.11,18,19 Although reliable when used by nonexpert nurses (inter-rater reliability mean 0.78), wound care expert (WOC nurse) use of the PSST resulted in higher reliability.16,17
Algorithm validity. The results of a content validity study1 of these algorithms indicated high overall content validity (CVI >0.8 for each section) but study participants expressed concerns about their use by nonexpert nurses and a need for more definitions. Reflecting on the absence of valid and reliable definitions in wound care, the experts themselves provided many different definitions for commonly used terminology. The items/concepts with the lowest CVI were inclusion of healing as the expected outcome and the management of wounds with >25% necrotic tissue/fibrin slough. To incorporate these concerns, the algorithms were expanded to include more specific recommendations and definitions and a computerized version was developed. Specifically, based on available guidelines of care, the components consideration of overall goals of care and need to address risk factors for nonhealing were moved to a more prominent location at the beginning of the algorithms and the importance of assessing wounds for signs of infection as well as the relationship between the presence of necrotic tissue and risk of infection was emphasized.20-24 Algorithms for wounds requiring debridement were expanded to include debridement options for acute and chronic wounds25-28 and risk factors for nonhealing were listed in the patient care plan.20,29,30 Finally, given the importance of wound monitoring and available evidence that wound size reduction after 2 to 4 weeks of care is a predictor of healing,31-33 this information was added to the “Expected Outcomes” section of the algorithms.
Subsequently, 21 wound care experts from various countries (physicians, nurses, and a physical therapist) participated in a content validation study of the updated algorithms.2 The overall CVI was similar to that of the original validation study (>0.8) with the exception of debridement options because non-US experts considered the use of wet-to-dry gauze for mechanical debridement substandard practice; this option was subsequently removed from the algorithms.
Algorithm usage preliminary outcome. The prospective validity of the algorithms has not been formally established but reports of outcomes when implemented in clinical practice have been published. Results of a prospective, cohort implementation study (N = 433 patients with 767 wounds) using the computerized version of the algorithms showed good outcomes in a variety of patient care settings.2 In a prospective controlled clinical study, Stage II and Stage III pressure ulcers managed using the algorithms to guide assessment and care (N = 29) had statistically significant (P <0.05) better healing outcomes as evidenced by a reduction in PSST score than patients whose ulcers were managed using traditional dressings (N = 34).34 Calculating costs, the researchers also reported that both cost-effectiveness (PSST score change divided by cost of care and material) and total costs for 12 weeks of care were significantly lower (P <0.05) in the algorithm-treatment group ($730.96 for algorithm and $1,671.53 non-algorithm group). Similarly, results of a noncontrolled study to assess the effects of implementing an algorithms-based software program in home care showed that the average time to healing acute and chronic wounds was reduced by 90% and costs of care were $946 lower per client per month.35 In 2008–2009, further support for each step of these algorithms was submitted to the Agency for Health Care Quality and Research as part of the evidence trail needed for inclusion of the updated guidelines on the National Guideline Clearing House website.1,2,17,20-77
Thus, while there is ample face validity, expert content validity, and some evidence that these algorithms promote optimal wound care (correct assessment and dressing selection), formal content and construct validity using nonexpert nurses have not been established and potential areas for improvement have not been identified.
Study Purpose
The purpose of this cross-sectional, mixed methods, quantitative study was to validate usage of the Solutions® Algorithms by nonexpert registered nurses. The research questions were: 1) How do registered nurses rate the appropriateness/validity of the components and processes inherent in the algorithms? 2) How well do registered nurses use the algorithms to select appropriate wound dressings with a variety of wounds? “How well” is measured by percent correct of appropriate selected algorithm and subsequent appropriate dressing choice. 3) What themes of positive responses or concerns about appropriateness do registered nurse users describe when assessing content validity and using the algorithms for wound care therapy selection? 4) What insights into wound care decision-making processes can be obtained from user comments about algorithm usage?
Methods
A cross-sectional, mixed methods, quantitative survey design was used to conduct the study. After receiving approval from a university’s Institutional Review Board (IRB), the researchers surveyed and contacted a purposive sample of practicing registered nurses associated with a School of Nursing and Health Sciences in the northeastern US with an urban and suburban satellite campuses. Data collection occurred over a period of 4 months at both locations. The survey and testing process were preceded by an explanation about the background and theoretical foundation of the algorithms, after which prospective participants were asked to sign the informed consent documents. Participants kept a copy of the form. Eligible participants had to be licensed registered nurses and able to speak, write, and understand English. Anonymity and confidentiality were assured — only code numbers (no personal identifiers) were used.
Instrumentation. A spiral-bound, paper copy of the algorithms was available for each study participant. Algorithm users start by considering wound moisture (see Figure 1), followed by amount of necrotic tissue in the wound bed (< 25% or > 25%). After making a selection on necrotic tissue amount, users flip the page to the algorithm selected and continue turning the required pages until they have made a dressing selection (average four pages).
A paper-pencil data collection instrument was designed with three segments: 1) demographic data; 2) content validation for the components pervading all eight algorithms; and 3) a photographic application activity for use with the algorithms. A final page requests qualitative comments from participants.
The demographic data section comprised 13 questions regarding attributes (age, gender), nursing certification (if any), highest level of education, years of nursing experience, practice setting, facility size, and wound care experience. The second section comprised 11 statements to assess participants’ opinions of the components pervading the eight algorithms related to: 1) assessment (amount of moisture and necrotic tissue, signs/symptoms of infection, wound depth, surrounding skin, wound edges, and delayed healing); 2) plan of care (goal, wound and patient care plan) and 3) expected outcomes. Each statement was accompanied by a 4-point rating scale: 1 = not relevant/appropriate, 2 = unable to assess relevance without revision, 3 = relevant but needs minor alteration, and 4 = very relevant and appropriate.8 After rating the relevance of each component to optimal wound care, participants were asked to write comments about statement omissions and provide suggestions for improvement or present an alternative.
The third section of the data collection instrument, the photographic application activity, comprised 15 test items. Each item required participants to look at a picture of a wound — projected using PowerPoint® slides (Microsoft Corporation, Seattle, WA) — to select the appropriate algorithm for its care and to write which product(s) would be appropriate given the algorithms consulted. Participants also could circle one or more wound assessment variables (wound depth, surrounding skin, wound edges) they considered important for their choice. The time taken by the group to complete each item was recorded. The photographic test activity was designed by the researchers to represent a variety of common wounds. Each of the 15 wound slides was labeled with a photograph number and the amount of moisture in the wound (eg, dry, heavily exudating). Each participant had to consider wound exudate condition (eg, minimal moisture = dry wound), assess the percent percentage of necrotic tissue present (< 25% or >25%), and consider which of the eight algorithmic pathways was most correct. Once selected, participants followed the algorithm to select and record the most appropriate dressing(s) to treat the wound.
The 15 depicted wounds included pressure ulcers, surgical wounds healing by secondary intention, venous ulcers, and vasculitic wounds in various anatomic locations. The wounds represented a variety of clean (five) wounds, wounds with necrotic tissue (10), and a variety of exudate (moisture) levels. Two pictures were repeats of the same photo to provide an opportunity for test-retest reliability measurement —ie, photograph 1 and photograph 8 were identical and photograph 2 and photograph 15 were identical.
The last page of the instrument provided an opportunity for participant input. Comments on overall study content and process and suggestions for improvement or added interventions were solicited.
Procedure. All nurses who agreed to participate received the algorithm book, to be returned at the end of the study. To simulate use of the algorithms without extensive inservicing, the algorithm format was explained for 5 to 10 minutes, data collection forms distributed, and participants were given an opportunity to ask questions about the study procedure only.
To rate the validity of the 11 critical components, participants were asked to examine all eight algorithms and complete the rating scores based on their past clinical experience. Next, each photo was displayed and participants were asked to 1) select the correct algorithm by writing in a number from one to eight; 2) record whether any special wound aspect was a trigger (eg, wound edges, surrounding skin); 3) select the appropriate dressing based on the algorithm selected and writing it in; and 4) add comments about each photograph exercise and any associated ease of choice or difficulty. At the end of the photos, participants completed the last page (overall comments) of the instrument.
Data collection and analysis. All data were collected on paper-pencil report forms. Quantitative data were coded and entered into an Excel version 2003 spreadsheet® (Microsoft Corporation) and uploaded into SPSS® version 16.0 (SPSS Inc, Chicago, IL) for analysis. Summary statistics were calculated for demographic data. Write-in demographic responses were tabulated. Mean scores for the 11 content components ratings were derived. The CVI was calculated by grouping items rated relevant/very relevant (ratings 3 and 4) and not relevant/unable to assess relevance (ratings 1 and 2).78 The proportion of items rated 3 or 4 (CVI) was calculated for each of the 11 components and all of the components as per Polit and Beck’s78 recommended clarification.
Participant selection of the correct algorithm for each wound photograph was calculated for percent correct of the primary (most correct) and secondary (acceptable and although not fully correct, resulted in selecting an appropriate dressing) algorithm choice. The proportion of participants who selected the correct primary and/or secondary dressings for the wound shown and the proportion who responded to “trigger” wound assessment variables (possibility of three choices) was also calculated. Percent correct choices also were analyzed in light of wound photographs with or without necrotic tissue in the wound bed. To ascertain reliability (repeatability) of algorithm usage, Pearson correlation coefficients were obtained for participants’ responses to the duplicate wound photograph slides (photograph 1 and photograph 8) and (photograph 2 and photograph 15).
A professional transcriptionist transcribed all participant responses and written comments. Using qualitative narrative data reduction techniques, researchers analyzed the typed transcripts for themes and associated subthemes. Sample indicator statements were related to obtained themes and subthemes. In addition to the researchers’ analyses, a doctoral-prepared nurse researcher with qualitative research experience reviewed the qualitative and quantitative data to check for completeness and clarity and to offer further insights. 
Results
Sample. All study participants were licensed registered nurses with varying educational levels (eg, diploma, associate, baccalaureate, master’s degree) and members of the RN-BSN program, MSN program, or nursing faculty. The total population of these programs/groups at the time of the study was 375; of those, 204 participated (54% response rate). The majority of participants (n = 180, 88%) were women, mean age of 34.8 years (SD 11.95, range 23 to 60), with between 4 to 9 years of nursing experience; 70 participants (34%) were certified in some nursing specialty (see Table I). Only three had earned some form of wound care certification. Most participants had a bachelor’s degree (n = 148, 73%). The group was relatively split in wound patient exposure; 100 participants (49%) saw between 0 and 49 wound patients in a year and 99 (48%) had contact with 50 or more wound patients yearly. The most commonly encountered types of wounds were pressure ulcers (n = 135), surgical wounds (n = 74), diabetic ulcers (n = 42), and venous ulcers (n = 37). Of the 204 participants, 22 reported formal wound care education: 18 had attended continuing education or hospital inservice programs. The majority of participants practiced in the acute care setting (n = 146, 72%); for the 28 respondents who reported “other” setting, the most common practice setting was an outpatient office.
Quantitative data. Quantitative data analysis suggests that registered nurse participants rated the algorithms generally valid and appropriate (research question 1). On a scale of 1 to 4, the mean scores for all 11 algorithms components ranged from 3.81 to 3.63. Only three of the components were rated below 3.70 (see Table 2). The CVI for the 11 components was strong — CVI results were >0.96 and the calculated total CVI for all 11 components was 0.98 (see Table 3). From these results, the algorithms can be interpreted as very relevant or in need of only minor revisions.
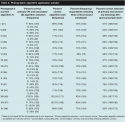
 Quantitative results also suggest that the algorithms help registered nurses match the wound to the appropriate primary algorithm and subsequently select the correct dressings for the displayed wound (research question 2). The percentage of correct primary algorithm selection ranged from 39% to 93%, with 71% to 98% of nurses selecting the correct (primary) or appropriate but not entirely correct (secondary) algorithm (see Table 4). The proportion of participants selecting the correct primary and, if appropriate, secondary dressing ranged from 75% to 91%. Thus, the structure of the algorithms had a protective effect in guiding users. The percent of correct algorithm selection increased when the related (nearly correct) secondary algorithm was included and led to a safe dressing choice for the vast majority of wounds shown. The proportion of nurses selecting the correct algorithm was higher for photographic wounds without necrotic tissue than for those with necrotic tissue. Necrotic wounds (10 photos) had an average 59% correct algorithm selection; non-necrotic wounds had an average of 80% (see Table 5).
Quantitative results also suggest that the algorithms help registered nurses match the wound to the appropriate primary algorithm and subsequently select the correct dressings for the displayed wound (research question 2). The percentage of correct primary algorithm selection ranged from 39% to 93%, with 71% to 98% of nurses selecting the correct (primary) or appropriate but not entirely correct (secondary) algorithm (see Table 4). The proportion of participants selecting the correct primary and, if appropriate, secondary dressing ranged from 75% to 91%. Thus, the structure of the algorithms had a protective effect in guiding users. The percent of correct algorithm selection increased when the related (nearly correct) secondary algorithm was included and led to a safe dressing choice for the vast majority of wounds shown. The proportion of nurses selecting the correct algorithm was higher for photographic wounds without necrotic tissue than for those with necrotic tissue. Necrotic wounds (10 photos) had an average 59% correct algorithm selection; non-necrotic wounds had an average of 80% (see Table 5).
Algorithm choices were generally significantly related (repeated) when the same photograph was shown again later. The correlation coefficients were r = .28 (P = 0.01) for wounds without necrotic tissue (photos 1 and 8) and r = .44 (P = 0.01) for wounds with necrotic tissue (photos 2 and 15).
The entire study lasted an average of 1 hour. Nurses spent 2 to 5 minutes looking at each slide, selecting and reviewing the algorithm, and completing the study instrument. Notably, required timing for slide assessment decreased as the activity progressed, a possible “practice” effect.
 Qualitative analysis. Eight major themes of positive response or concerns were generated from the narrative analysis (see Table 6). In general, participants found the algorithms helpful, educational, and instructive (research question 3). Respondents thought the algorithms made wound assessment easier, that their inherent structure was logical and easy to follow, and that the photographs in the algorithms were a great assistance. Participants believed the algorithms would aid consistency of care and help with wound care planning. Many respondents asked for a copy of the algorithms book for learning purposes. Overall, participants enjoyed the research process and identified correct structural design issues (eg, duplicate pictures). A need for more education about wound assessment, wound products, and general use of the algorithms was frequently mentioned.
Qualitative analysis. Eight major themes of positive response or concerns were generated from the narrative analysis (see Table 6). In general, participants found the algorithms helpful, educational, and instructive (research question 3). Respondents thought the algorithms made wound assessment easier, that their inherent structure was logical and easy to follow, and that the photographs in the algorithms were a great assistance. Participants believed the algorithms would aid consistency of care and help with wound care planning. Many respondents asked for a copy of the algorithms book for learning purposes. Overall, participants enjoyed the research process and identified correct structural design issues (eg, duplicate pictures). A need for more education about wound assessment, wound products, and general use of the algorithms was frequently mentioned.
Many participants noted a personal deficit in understanding wound terminology, wound assessment skills, and knowledge of wound products, which, in turn affected their decision-making process (research question 4). Multiple participants noted difficulty in differentiating necrotic tissue from healthy tissue. Several discussed difficulty recognizing yellow or tan tissue (slough) as soft necrotic tissue versus eschar that was easier to identify. Participants also noted lack of familiarity with wound terminology (slough versus eschar, induration, superficial versus partial-thickness).
 An interesting finding was the effect of pressure ulcer education on participants’ wound assessment. Many participants noted the difficulty of considering wounds and wound therapy based on moisture level and percentage of necrosis instead of stage. Staging had created such a strong impression on participants’ approach to wound care that they struggled with care when it was not included and found the concept hard to disregard. Although participants most commonly described experience managing pressure ulcers, many had experience caring for patients with other types of wounds. Several participants described the study as a “learning experience” to examine wounds in light of partial- versus full-thickness depth of tissue damage.
An interesting finding was the effect of pressure ulcer education on participants’ wound assessment. Many participants noted the difficulty of considering wounds and wound therapy based on moisture level and percentage of necrosis instead of stage. Staging had created such a strong impression on participants’ approach to wound care that they struggled with care when it was not included and found the concept hard to disregard. Although participants most commonly described experience managing pressure ulcers, many had experience caring for patients with other types of wounds. Several participants described the study as a “learning experience” to examine wounds in light of partial- versus full-thickness depth of tissue damage.
Other important themes included observations that although the algorithms were helpful and educational, many believe their use should be explained in more detail through educational and inservice programs (see Table 6). It also was noted that algorithm use became easier and proceeded faster with practice and that a one-page per algorithm format would be helpful. The researchers observed considerable variations in time needed to identify the correct algorithm and complete the required information. Some nurses consistently completed every wound assessment in a couple of minutes; others required at least 5 minutes for every slide. Most participants identified all three individual wound characteristics (eg, wound depth, surrounding skin, wound edges) rather than any individual or pair of characteristics as important in dressing choice. Further analysis of wound characteristics per algorithmic type is beyond the scope of the present study but is planned by the researchers. 
Discussion
Content validity. The overall CVI of this set of algorithms as rated by the 204 registered nurses in the study was strong and slightly higher than the CVI obtained when the instruments were tested by wound care experts.1,2 The statements made by current study participants about the value of these algorithms and the finding that algorithms and other methods of organizing large amounts of data are most helpful for nonexpert healthcare professionals3 may explain these results. Individual ratings of the 11 components that pervade the eight algorithms also were high, suggesting they do not require changes. Confidence in the validity of the findings is further increased by the large sample size. Although still considered valid (CVI > 0.8),78 components with a lower score included identifying goals of wound care and expected outcomes as well as the need to choose an appropriate patient care plan and evaluate for delayed healing. Because these are the only components regarding provision of optimal wound care that do not appear to directly affect which dressing or treatment to choose, it seems logical that nonexpert nurses would not consider these variables as important as, for example, signs and symptoms of infection.
Construct validity and nurses’ knowledge. Standards of local wound care have remained relatively unchanged for the past few decades and include a complete wound assessment, debridement as needed, cleansing, and maintenance of a moist wound environment.15,79-82 The provision of appropriate local care is contingent on an accurate wound assessment, including wound location, exudate amount, depth, extent (size), undermining, tunneling, condition of surrounding skin and wound edges, infection, and wound bed appearance.83
The amount and characteristics of devitalized tissue is an important variable that determines the need for, and best method to, accomplish, debridement.26,27 The participants in the study noted their inability to quickly identify necrotic tissue (eschar, slough) so an appropriate algorithm and topical wound therapy would be selected. In the study, the proportion of appropriate decisions made was generally high but notably lower for necrotic wounds. Also, while the reliability coefficients for the two sets of two slides were modestly good, the coefficient for wounds with necrotic tissue was surprisingly higher than that for clean wounds. These findings confirm that wound assessment skills affect nurses’ ability to select an appropriate treatment modality consistently or effectively.
Exudate amount also affects the type of dressing selected. Because exudate amounts cannot be seen on slides, participants were told the amount; hence, this variable was not tested in a complete sense. Despite these challenges, the proportion of correct algorithm choice ranged from 39% to 93%; whereas, the proportion of correct treatment and dressing choices ranged from 75% to 91%. This suggests that the use of valid algorithms, such as those tested, may have a protective effect in guiding users with limited assessment skills.
The proportion of correct wound care choices in this study also was higher than reported in the literature. Research by Melchior-MacDougall and Lander84 using wound photographs and a panel of 94 home care nurses showed that the percentage of nurses who made appropriate wound treatment decisions was significantly higher when nurses used a wound management decision tree (28% versus 23% when no decision tree was provided). Nevertheless, the proportion of appropriate decisions made was low and the proportion of most appropriate decision was even lower (12%). In addition to the inherent limitations of using photographs to make treatment decisions, which also may have affected the study results, the researchers also noted that the use of decision trees is not a substitute for education.
Buckley et al,85 using digital images to ascertain the ability of registered nurses (n = 33) to correctly assess wound bed color, also observed that the percent of correct answers decreased as the appearance of the wound bed became more “complex.” More than 89% of wounds with one color were assessed correctly, compared to <75% of wounds with two and <25% of wounds with three or four colors.
Interestingly, Buckley et al85 were unable to obtain 100% agreement among wound expert (WOC) nurses when validating wound terminology. In addition to some limitations in using photographs to assess wounds, they noted a poor understanding of commonly used wound terms. In another study86 that used real wounds instead of photographs, other researchers asked eight nurses and eight physicians to complete the hospital’s standard wound assessment form. Two physicians and four nurses recognized the necrotic tissue present in the wound and 50% of study participants indicated they did not know if granulation tissue was present.
Implications for education and research. Results of the current study confirm that common, valid, and reliable wound definitions are needed, especially because most wound care is provided by non-specialty healthcare professionals. A 1994 survey87 of extended care facilities showed that 90% of wound care was provided by licensed practical nurses and nonspecialty physicians made 65% of their facilities’ product decisions (mostly for the use of gauze-type dressings). When Zeleznik et al88 reviewed the charts of 75 patients with osteomyelitis and at least one ulcer, they found that not only was wound documentation incomplete, but also that house staff and attending physicians used 66 unique terms, including 38 nonmedical, nonspecific, or ambiguous terms, to describe the wounds.
Nonspecialty, licensed, healthcare professionals receive limited wound education. Total hours of required wound education received in medical schools has been documented to average 9.2 hours in the US, 4.9 in the UK, and 9 hours in Germany.89 Research regarding nursing education vis-à-vis pressure ulcers has been conducted but general wound care knowledge and education research are limited. Results of a survey90 conducted among readers of Nursing 2004 (N = 692) showed that only 30% of nurses believed their basic education program had provided them with sufficient education about chronic wounds. A recent survey91 about clinicians’ wound healing knowledge and practice reiterated the same message about understanding of wound care — 74 physicians and nurses reported wound care practices that demonstrated a lack of evidence-based care and outdated approaches. Wound care knowledge was primarily obtained from colleagues and personal experience rather than the literature.
Other studies demonstrate various perspectives on the same underlying themes of inadequate clinician knowledge and lack of standardization of wound terminology and care approaches. Dunk and Taylor92 surveyed nurses and midwives about care of infected wounds focusing on use of terminology and products. The 178 respondents noted that they used wound assessment documentation forms for an infected wound <50% of the time. More expert clinicians used the term cellulitis as the most important indicator of infection, while less expert clinicians used words such as odor and pain. More than 50% of respondents based product use for infected wounds on colleagues’ opinion rather than the literature. The researchers reiterated the need for an evidence-based approach to wound care, more education about product choices, and a common language for clinical indicators. Other studies have found similar outcomes. For physicians,93 critical care nurses,94 and others,95 research analyses have demonstrated a need for substantively improved wound assessment and care education and a need for ongoing education to maintain clinical currency.
Implications for practice. Although wound education improvements in all disciplines are needed, the results of this study confirm that algorithms help nonexpert nurses’ decision-making.5 Because excellent clinical outcomes using these algorithms in a variety of clinical settings have been reported3,34,35 and because the effects of optimal wound care on healing and costs of managing acute and chronic wounds has been well established,60,61,65 the guidance provided by these algorithms may help improve patient outcomes by addressing documented wound care knowledge deficits and assessment inconsistencies among nonexpert nurses and physicians.96,97
Limitations and implications. The sample included registered nurse respondents practicing mostly in one metropolitan and geographic area of the country. Because participants were currently enrolled students and faculty, they may not be fully representative of nursing staff who have not furthered their education. Conversely, the sample was highly representative of registered nurses in that the majority had little or no formal wound care education. The method has limits as well. Survey design, even with mixed method narrative (qualitative) input, may not provide an indepth of understanding of the clinical decision-making process.
The present study did address some limitations of the previous study1 in that staff nurses (the intended users) provided content and construct validity data (using algorithms with real wound care situations) for the specified algorithms — ie, they found algorithm components appropriate and selected safe wound care intervention. Although these algorithms generally promoted patient safety in guiding users to correct therapy selection, the results also suggest that basic wound assessment knowledge factors and insecurity about wound assessment interfered with optimal use. Because the algorithms are based on percent of necrotic tissue in a wound bed, it is imperative that users (eg, nurses) recognize normal versus devitalized tissue. In addition, when the algorithm is used to guide care, issues with reading ability/speed arose. In the narrative comments and in conversations following data collection, several students described lack of familiarity and comfort with algorithms. Researchers noted that for many students verbalizing confusion, English was not their native language. Given the nature of the data collection, it is impossible to know if their basic nursing education occurred in the US or elsewhere. Several participants identified reading speed issues in using the algorithms.
Another limitation is related to the data collection process. First, because wound moisture amounts could not be simulated, each picture had the amount of moisture (dry, lightly exudating, heavily exudating) clearly described. In practice, this judgment has to be made by the clinician. Second, study participants had very little time to review the algorithms before use and did not have the benefit of an inservice or educational program. Even though this may reflect a common scenario in clinical practice, this is not an ideal situation and many study participants commented that educational and inservice programs are needed for optimal algorithm implementation. Consequently, the results of this study may present the “lowest one can expect” accuracy ratings.
Future construct validity and reliability research should include an inservice program before data collection and more demographic background variables such as country of origin.
Conclusion
Quality wound care is a major concern for clinicians in all patient care environments. Algorithms have been developed to expedite the clinical decision-making process. Ideally, when used by both expert and nonexpert staff (eg, staff registered nurses) algorithms should guide optimal prevention efforts and clinical care delivery. DeVon et al98 submit that the foundation of a rigorous instrument or scale (such as the algorithm in this study) is confirmation of its validity and reliability. Construct validity is the degree to which an instrument or tool measures the construct (eg, appropriate wound care) that it is intended to measure and is based on translational validity (face and content) and criterion validity (concurrent, predictive, congruent or discriminant). The results of this study support that these algorithms have face validity, are valid for their content validity, and have construct validity in terms of predictive validity usage in promoting optimal care with a variety of wound types and that they do so reliably in a test, re-test format. In other words, this study provides some evidence that wound care algorithms promote safe, effective wound care.
The results of this study also suggest that multiple factors may affect their optimal use. Most critically, users identified their lack of wound assessment education (eg, assessment of necrotic versus healthy tissue) as a barrier to best use, an observation supported by the quantitative results of this study. Consequently, algorithms designed for improving patient care must be used by clinicians educated in their particular use; optimal use cannot be expected unless instruction has been provided in basic algorithm function and focus — fundamentals of wound assessment and wound care.
1. Beitz JM, van Rijswijk L. Using wound care algorithms: a content validation study. JWOCN. 1999;26:238–249.
2. Bolton L, McNees P, van Rijswijk L, et al. Wound healing outcomes using standardized assessment and care. JWOCN. 2004;31(2):65–71.
3. Gaines C. Concept mapping and synthesizers: instructional strategies for encoding and recalling. JNY State Nurses Assoc. 1996;27:14–18
4. Hadorn DC, McCormick K, Diokno A. An annotated algorithm approach to clinical guideline development. JAMA. 1992;267:3311–3314.
5. Beitz J. Concept maps: navigating the learning process. Nurse Educator. 1997;23(5):35–41.
6. Mouës CM, Heule F, Legerstee R, Hovius SER. Five millennia of wound care products – what is new? A literature review. Ostomy Wound Manage. 2009;55(3):16–32.
7. Grant JS, Davis LL. Selection and use of content experts for instrument development. Res Nurs Health. 1997;20:269–274.
8. Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35:382–385.
9. Solutions wound care algorithm. ConvaTec June 2005. Available at: www.guideline.gov/summary/summary.aspx?doc_id=8534&nbr=4749&ss=6&xl=999. Accessed May 12, 2009.
10. Waltz CW, Bausell RB. Nursing Research: Design Statistics and Computer Analysis. Philadelphia, PA: FA Davis;1981.
11. Bates-Jensen BM, Vredevoe D, Brecht ML. Validity and reliability of the Pressure Sore Status Tool. Decubitus. 1992;5(6):20–28.
12. Cavorsi J, Vicari F, Wirthlin D, et al. Best-practice algorithms for the use of a bilayered living cell therapy (Apligraf®) in the treatment of lower-extremity ulcers. Wound Rep Regen. 2006;14:102–109.
13. Cutting KF, White R. Defined and refined: criteria for identifying wound infection revisited. Br J Community. 2004;9(3):S6–S15.
14. LeGood R. Pressure ulcers: guideline development and economic modeling. J Advanced Nurs. 2005;50(8):307–314.
15. Baranoski S. Wound assessment and dressing selection. Ostomy Wound Manage. 1995;41(suppl 7A):7S–12S.
16. Braden B, Bergstrom N. The Braden Scale for predicting pressure sore risk. Available at www.bradenscale.com/bradenscale.htm. Accessed May 26, 2009.
17. Kantor J, Margolis DJ. Efficacy and prognostic value of simple wound measurements. Arch Dermatol. 1998;134:1571–1574.
18. Bates-Jensen B, Sussman C. Tools to measure wound healing. In: Sussman C, Bates-Jensen B (eds). Wound Care: A Collaborative Practice Manual for Health Professionals. Baltimore, MD: Lippincott, Williams & Wilkins;2007:123–169.
19. Bates-Jensen BM, McNees, P. Toward an intelligent wound assessment system. Ostomy Wound Manage. 1995;41(suppl 7A):1S–7S.
20. Kerstein MD. The non-healing leg ulcer: peripheral vascular disease, chronic venous insufficiency and ischemic vasculitis. Ostomy Wound Manage. 1996;42(10A suppl):19S–35S.
21. de Laat EH, Scholte op Reimer WJ, van Achterberg T. Pressure ulcers: diagnostics and interventions aimed at wound-related complaints: a review of the literature. J Clin Nurs. 2005;14(4):464–472.
22. Dow G. Bacterial swabs and the chronic wound: when, how, and what do they mean. Ostomy Wound Manage. 2003;49(5 suppl A):8–13.
23. Gardner SE, Frantz RA, Doebbeling BN. Validity of the clinical signs and symptoms used to identify chronic wound infection. Wound Rep Regen. 2001;9:178–186.
24. Brem H, Sheehan P, Rosenberg HJ,Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg. 2006;117(7S):193S–205S.
25. Sieggreen MY, Maklebust J. Debridement: choices and challenges. Adv Wound Care. 1997;10:32–37.
26. National Institute Clinical Excellence. Guidance on the use of debriding agents and specialty wound care clinics for difficult to heal surgical wounds. Technology Appraisal # 24. NICE 2001. Available at: www.nice.org.uk. Accessed September 1, 2009.
27. Bradley M, Cullum N, Sheldon T. The debridement of chronic wounds: a systematic review. Health Tech Assess. 1999;3(17 Part 1):1–78.
28. Edwards J. Debridement of diabetic foot ulcers. The Cochrane Collaboration. Available at: http://thecochranelibrary.com. Accessed March 26, 2010,
29. Association for the Advancement of Wound Care (AAWC). Summary algorithm for venous ulcer care, National Guideline Clearinghouse, July 29, 2005. Available at: www.guideline.gov. Accessed March 26, 2010.
30. O’Meara S, Cullum N, Nelson EA. Compression for venous leg ulcers. Cochrane Review. The Cochrane Library 2009. Available at: http://thecochranelibrary.com. Accessed March 20, 2010.
31. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol. 2000;142:960–964.
32. van Rijswijk L, Polansky M. Predictors of time to healing deep pressure ulcers. Wounds. 1994;6(5):159–165.
33. Sheehan P, Jones P, Caselli A, Giurini J, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26(6):1879–1882.
34. Ohura T, Sanada H, Mino Y. Clinical activity-based cost effectiveness of traditional versus modern wound management in patients with pressure ulcers. WOUNDS. 2004;16(5):157–163.
35. McIsaac C. Managing wound care outcomes. Ostomy Wound Manage. 2005;51(4):54–59.
36. United States Food and Drug Administration. Guidance for Industry – chronic cutaneous ulcer and burn wounds – developing products for treatment. June 2006. Available at: www.fda.gov/cder/guidance/index.htm. Accessed March 26, 2010.
37. National Institute for Clinical Excellence. Surgical site infection: full guideline (segment on debriding agents) 2008. Available at: www.nice.org.uk. Accessed March 26, 2010.
38. Adam DJ, Naik J, Hartshorne T, Bello M, London NJ. The diagnosis and management of 689 chronic leg ulcers in a single-visit assessment clinic. Eur J Vasc Endovasc Surg. 2003;25(5):462–468.
39. AHCPR Panel for the Prediction and Prevention of Pressure Ulcers in Adults. Pressure ulcers in adults: Prediction and prevention. Clinical Practice Guideline, No. 3. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research. May, 1992. AHCPR Publication No. 92-0047.
40. Alexanderhouse Group. Consensus paper on venous leg ulcers. Phlebology. 1992;7:48–58.
41. Alvarez OM, Fernandez-Obregon A, Rogers RS, et al. A prospective, randomized, comparative study of collagenase and papain-urea for pressure ulcer debridement. Wounds. 2002;14:293–301.
42. Arnold TE, Stanley JC. Prospective, multicenter study of managing lower extremity venous ulcers. Ann Vasc Surg. 1994;9(4):356–362.
43. Barnea Y, Amir A, Leshem D, et al. Clinical comparative study of AQUACEL and paraffin gauze dressing for split-skin donor site treatment. Ann Plast Surg. 2004;53(2):132–136.
44. Bolton LL. Evidence-based report card: which pressure ulcer risk assessment scales are valid for use in the clinical setting? JWOCN. 2007;34(4):368–381.
45. Boulton AJ Meneses P, Ennis WJ. Diabetic foot ulcers: a framework for prevention and care. Wound Rep Regen. 1999;7:7–16.
46. Bouza C, Saz Z, Muñoz A, Amate JM. Efficacy of advanced dressings in the treatment of pressure ulcers: a systematic review. J Wound Care. 2005;14(5):193–199.
47. Burgos A, Giminez J, Moreno EK, et al. Cost efficacy, efficiency and tolerability of collagenase ointment versus hydrocolloid occlusive dressing in the treatment of pressure ulcers: a comparative, randomized, multicentre study. Clinical Drug Invest. 2000;19(5):357–365.
48. Chaby G, Senet P, Vaneau M, Martel P, Guillaume JC, Meaume S, et al. Dressings for acute and chronic wounds: a systematic review. Arch Dermatol. 2007;143(10):1297–1304.
49. Charles H, Callicot C, Mathrin D, Ballard K, Hart J. Randomized, comparative study of three primary dressings for the treatment of venous ulcers. Br J Community Nurs. 2002;7(6):48–52.
50. Cordts PR, Hanrahan LM, Rodriguez AA, et al. A prospective, randomized trial of Unna’s boot versus Duoderm CGF hydroactive dressing plus compression in the management of venous leg ulcers. J Vasc Surg. 1992;15:480–486.
51. Daniels S, Sibbald RG, Ennis W, Eager CA. Evaluation of a new composite dressing for the management of chronic leg ulcer wounds. J Wound Care. 2002;11(8): 290–294.
52. Duby T, Cherry G, Hoffman D, Cameron J, Dobloff-Brown DK, Ryan T. A randomized trial in the treatment of venous leg ulcers comparing short stretch bandages, four layer bandage system, and a long stretch-paste bandage system. Wounds. 1993;5(6):276–279.
53. Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006;14(5):548–557.
54. Goetze S, Ziemer M, Kaatz M, Lipman RD, Elsner P. Treatment of superficial surgical wounds after removal of seborrheic keratoses: a single-blinded randomized-controlled clinical study. Dermatol Surg. 2006;32(5):661–668.
55. Harding KG, Price P, Robinson B, Thomas S, Hofman D. Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community-based patients with chronic leg ulceration. Wounds. 2001:13(6):229–236.
56. Heffernan A, Martin AJ. A comparison of a modified form of granuflex (Granuflex Extra thin) and a conventional dressing in the management of lacerations, abrasions, and minor operation wounds in an accident and emergency department. J Acc Emerg Med. 1994;11:227–230.
57. Hopf H, Ueno C, Aslam R, et al. Guidelines for treatment of arterial insufficiency ulcers. Wound Rep Regener. 2006;14:693–710.
58. Jorgensen B, Price P, Andersen KE, et.al. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonized venous leg ulcers: a randomized, controlled trial. Int Wound J. 2005;2(1):64–73.
59. Jude EB, Apelqvist J, Spraul M, Martini J and the Silver Dressing Study Group. Prospective randomized controlled study of Hydrofiber® dressing containing ionic silver or calcium alginate dressings in non-ischaemic diabetic foot ulcers. Diabetic Med. 2007;24:280–288.
60. Kerstein MD, Gemmen E, van Rijswijk L, et al. Cost and cost effectiveness of venous and pressure ulcer protocols of care. Dis Manage Health Outcomes. 2001;9(11):651–636.
61. Kobza L, Scheurich A. The impact of telemedicine on outcomes of chronic wounds in the home-care setting. Ostomy Wound Manage. 2000;45(10):48–53.
62. Koksal C, Bozkurt AK. Combination of hydrocolloid dressing and medical compression stocking versus Unna’s boot for the treatment of venous leg ulcers. Swiss Med Wkly. 2003;133:364–368.
63. Laing PW, Cogley DI, Klenerman L. Neuropathic foot ulceration treated by total contact casts. J Bone Joint Surg (Br). 1991;74:133–136.
64. Mak S, Molassiotis A, Wan W, Lee IYM, Chan ESJ. The effects of hydrocolloid dressing and gentian violet on radiation-induced moist desquamation wound healing. Cancer Nurs. 2000;23(3):220–229.
65. Madden M, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudates upon keratinocytes proliferation. J Trauma. 1989;29(7):924–930.
66. Mulder GD. Cost-effective managed care: gel versus wet-to-dry for debridement. Ostomy Wound Manage. 1995;41:68–70.
67. Nemeth A, Eaglstein WH, Taylor JR, Peerson LJ, Falanga V. Faster healing and less pain in skin biopsy sites treated with an occlusive dressing. Arch Dermatol. 1991;127:1679–1683.
68. Murharyo P. Dressings following circumcision: results of a controlled clinical study. Singapore Paediatric J. 1996;38(3):125–130.
69. Phillips TJ, Machado F, Trout R, Porter J, Olin J, Falanga V, the Venous Ulcer Study Group. Prognostic indicators of venous ulcers. J Am Acad Dermatol. 2000;43:627–630.
70. Registered Nurses’ Association of Ontario. Assessment and management of foot ulcers for people with diabetes. Available at: www.guideline.gov. Accessed at September 25, 2008.
71. Saap LJ, Falanga V. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. Wound Rep Regen. 2002;10:354–359.
72. Steed DL, Donohoe D, Webster MW, et al. Effect of extensive debridement on the healing of diabetic foot ulcers. J Am College Surg. 1996;183:61–64.
73. Thomson PD, Smith DJ. What is infection? Am J Surg. 1994;167(1A suppl):7S–11S.
74. Tan ST, Roberts RH, Sinclair SW. A comparison of Zenoderm® with DuoDERM E® in the treatment of split skin graft donor sites. Brit J Plast Surg. 1993;46:82–84.
75. van Rijswijk L, the Multi-Center Leg Ulcer Study Group. Full-thickness leg ulcers: patient demographics and predictors of healing. J Family Pract. 1993;36(6):625–632.
76. Wiechula R. The use of moist wound-healing dressings in the management of split-thickness skin graft donor sites: a systematic review. Int J Nurs Pract. 2003;9:S9–S17.
77. Tesfaye S, Chaturvedi N, Eaton SEM, et al. Vascular risk factors and diabetic neuropathy. New Eng J Med. 2005;352:341–350.
78. Polit DF, Beck CT. The content validity index: are you sure you know what’s being reported? Critique and recommendations. Res Nurs Health. 2006;29:489–497.
79. United States Food and Drug Administration. Guidance for Industry – chronic cutaneous ulcer and burn wounds – developing products for treatment. July 2003. Available at: www.fda.gov/cder/guidance/index.htm. Accessed March 26, 2010.
80. National Guideline Clearinghouse. Guideline synthesis: management and treatment of pressure ulcers. Available at: www.guideline.gov. Accessed March 26, 2010.
81. Association for the Advancement of Wound Care (AAWC). Summary algorithm for venous ulcer care with annotations of available evidence. Malvern (PA): 2005. Available at: www.guideline.gov. Accessed March 27, 2010.
82. National Institute for Clinical Excellence. Guidance on the use of debriding agents and specialist wound care clinics for difficult to heal surgical wounds. April 2001. Available at: www.nice.org.uk. Accessed March 27, 2010.
83. van Rijswijk L, Catanzaro J. Wound assessment and documentation. In: Krasner DL, Rodeheaver GT, Sibbald RG, eds. Chronic Wound Care: A Clinical Sourcebook for Healthcare Professionals, 4th ed. Malvern, PA. HMP Communications;2007;113–126.
84. Melchior-MacDougall F, Lander J. Evaluation of a decision tree for management of chronic wounds. JWOCN. 1995;22:81–88.
85. Buckley KM, Tran BQ, Adelson LK, Agazio JG, Halstead L. The use of digital images in evaluating home care nurses’ knowledge of wound assessment. JWOCN. 2005;32(5):307–316.
86. Stremitzer, S, Wild T, Hoelzenbein, T. How precise is the evaluation of chronic wounds by health care professionals? Int Wound J. 2007;4:156–161.
87. Ballard-Krishnan S, van Rijswijk L, Polansky M. Pressure ulcers in extended care facilities: Report of a survey. J WOCN. 1994;21:4–11.
88. Zeleznik J, Agard-Henriquez B, Schnebel, B. Terminology used by different health care providers to document skin ulcers; the blind men and the elephant. J WOCN. 2003;30:324–333.
89. Patel, NP, Granick, MS, Kanakaris, NK,. Giannoudis, PV et al. Comparison of wound education in medical schools in the United States, United Kingdom, and Germany. E plasty, January 8, 2008. Available at: www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2205997. Accessed May 26, 2009.
90. Ayello E, Baranoski S, Salati DS. Wound care. Nursing. 2005:35(5):36–45.
91. Ashton J Price P. Survey comparing clinicians wound healing knowledge and practice. Br J Nurs. 2006;15(19):518–526.
92. Dunk AM, Taylor J. A survey of clinicians’ perceptions of and product choices for the infected wound. Wound Pract Res. 2009;17(1):5–11.
93. Odierna E, Zeleznik J. Pressure ulcer education: a pilot study of the knowledge and clinical confidence of geriatric fellows. Adv Skin Wound Care. 2003;16(1):26–30.
94. Tweed C, Tweed M. Intensive care nurses’ knowledge of pressure ulcers: development of an assessment tool and effect of an educational program. Am J Crit Care. 2008;17(4):338–347.
95. Vermeulen H, Ubbink DT, Sckreuder SM, Lubbers MJ. Inter-and intra-observer (dis)agreement among nurses and doctors to classify colour and exudation of open surgical wounds according to the red-yellow-black scheme. J Clin Nurs. 2007;16:1270–1277.
96. Pieper B, Templin T, Dobal M, Jacox A. Home care nurses’ ratings of appropriateness of wound treatments and wound healing. J WOCN. 2002;29:20–28.
97. Lamond D, Farnell S. The treatment of pressure sores: a comparison of novice and expert nurses’ knowledge, information use, and decision-making accuracy. J Adv Nurs. 1998;27:280–286.
98. DeVon HA, Block ME, Moyle-Wright P, et al. A psychometric toolbox for testing validity and reliability. J Nurs Scholarship. 2007;39(2):155–164.














