New Year, Old Challenge: Pressure Injuries
Introduction
As 2021 begins, the data on 2020 hospital-acquired conditions throughout the United States are being reviewed. What a year it was ... full of the unknown, sorrow, and losses, but also incredible courage, resilience, care, and dedication. The topic of pressure injuries (PIs) was and is still relevant, and the coronavirus disease-19 (COVID-19) pandemic posed challenges to the assessment and prevention of hospital-acquired PIs. Shortages of personnel, fear of COVID-19 transmission, interfering personal protective equipment, and critically ill patients made already challenging tasks even harder.
Pediatric hospitals were not excluded from the challenges. As spring 2020 melted winter snow, a hurricane of critically ill patients sought treatment. Northwell Medical System treated the largest number of COVID-19–positive patients in New York State in 2020.1 Pediatric intensive care units (ICUs) housed COVID-positive children, often critically ill with multisystem inflammatory syndrome and cardiac, respiratory, and kidney failure, leading to intubation and immobility. Neonatal ICUs admitted dozens of neonates born to COVID-positive parents and provided care for many patients with limited personnel because of concerns about exposure. Elective surgeries were cancelled, and those children who did require surgery were fragile and ill.
As Northwell’s 3 main hospitals filled with adult patients (even filling multiple floors in the pediatric hospital), health care providers changed as well. Many pediatric providers were reassigned to adult patients; as nurses from pediatric units contracted COVID-19 or quarantined, nurses from other units and even other institutions provided care in pediatric and neonatal ICUs. Despite these unprecedented conditions, Northwell’s pediatric hospital saw a decrease in the number and severity of PIs. The challenges that were faced due to the pandemic were shared by pediatric hospitals all over the United States and the world.
The SPS Network
The Children’s Hospitals’ Solutions for Safety Network (SPS Network) works to improve pediatric patient safety and eliminate harm to children who are admitted to the hospital. Started by the Ohio Children’s Hospital Association in 2009, Ohio State officials, the Ohio Department of Health, and 8 Ohio children’s hospitals joined together to establish the Ohio Children’s Hospitals Solutions for Patient Safety Network to focus on pediatric quality improvement projects. In 2011, following the success in Ohio, 25 hospitals from outside that state joined the group to launch the SPS Network and implement quality improvement and patient safety strategies across the country. Cohen Children’s Medical Center of NY was one of those institutions. In 2015, the Network grew to encompass more than 100 children’s hospitals and expanded into Canada. Leaders from these hospitals have committed to clear, shared goals of harm reduction. The SPS Network focuses on 11 specific hospital-acquired conditions, including PIs, and also is 1 of 16 members of the Hospital Improvement Innovation Networks. Midway through 2020, the SPS Network reinvigorated the monthly PI group meetings. These virtual meetings have broadened the horizon, welcomed increased participation, and promoted a culture of sharing. Virtual conference rooms have been filled with dedicated practitioners from dozens of children’s hospitals sharing, comparing, planning, and learning from each other.
PRESSURE INJURY ETIOLOGY
The traditional understanding of the common causes of PI entails the presence of pressure, shear, friction, immobility, and microclimate.1,2 The common pathway to injury is ischemia. Prolonged pressure causes microcirculatory occlusion, leading to poor oxygenation, tissue death, and ulceration. Tissue injury is thought to be a secondary process due to ischemia. A contemporary understanding focuses on sustained cell and tissue deformation as a triggering event and primary cause of tissue damage.3,4 Cell injury is the primary process, rather than the culmination of various injurious forces. This is an important point; by the time tissue injury is visible to the naked eye, the inner cells are already affected because physiologic damage started from the inside out, not from the outside in.
Inflammatory changes occur in cells exposed to sustained force and deformation. Cytoskeleton and cell wall injury lead to cell breakage, internal content leakage, mitochondrial dysfunction, energy generation failure, and transition to anaerobic metabolism. Toxic metabolic waste accumulates, further contributing to cell necrosis and apoptosis; lactate accumulation leads to the body’s attempt at physiologic hyperemia (vasodilation of blood vessel), which is not possible if continuous pressure is applied, leading to compressed blood vessels. Lack of disposal of toxic metabolites due to lymphatic dysfunction leads to further inflammation, oxidative stress, and tissue injury. Edema and interstitial fluid accumulation, along with subepidermal tissue damage, will eventually lead to visible tissue changes: stage 1, if only mild subepidermal injury occurred and reactive hyperemia is successful at restoring blood flow; stage 2, if epidermal/dermal tissues were injured; and stages 3 and 4, if injury progressed further inward and involved deeper tissues. This injury can be potentiated if pressure is lifted temporarily, leading to reperfusion injury; oxygen radicals, toxic metabolites, and microthrombi are propagated further, leading to secondary cell damage. Muscle damage can occur as well, especially with prolonged damage and at the bone-muscle interphase.
Many factors contribute to the degree of deformation. Prolonged and sustained pressure, poor vascularity (eg, columellar area or ear cartilage), and compression against a bony prominence sustain pressure. Friction distorts tissue, resulting in shear forces and contributing to worsening tissue injury. A common example is a prone neonate with a nasal cannula who is experiencing friction between the mattress and skin as well as the nasal cannula and skin. At times the body may slide, leading to shear forces between the skin and the plastic tubing. Microclimate (temperature, humidity, and moisture content) of the area may weaken the skin, potentiating the already injured area from pressure and shear.3-5 Noninvasive oxygen-delivering devices contribute to increased moisture and temperature around the skin, increase the coefficient of friction, weaken the skin by lowering the threshold for inflammation, and lead to earlier PI formation. Other factors, such as immobility, altered sensation, or inability to verbalize pain, contribute to PI burden. Inherent features of neonatal skin, such as poor epidermal/dermal adhesion as well as dermal and subcutaneous tissue thinness, potentiate injury.5 High metabolic rate, upregulated desquamation, and propensity for edema weaken the skin as well. Poor nutrition, organ failure, and mental state may play a role in older children.5
Devices
All medical devices in contact with the skin can predispose to PIs.6 As I reviewed my hospital’s data for 2020, the following devices emerged as causing the highest number of PIs: noninvasive respiratory devices, intravenous catheters, electroencephalogram leads, and pulse oximeters. Based on conversations during monthly SPS Network virtual meetings (attended by doctors, nurses, physical therapists, wound specialists, quality representatives, and any other provider related to hospitals’ PI prevention team), respiratory devices and electroencephalogram leads also seemed to dominate the larger pediatric PI arena; immobility leading to sacral injuries in older, critically children and extracorporeal membrane oxygenation-related PI were prevalent country-wide as well.
Although frequent skin assessment and body repositioning remain at the helm of PI prevention,5,6 these interventions are insufficient with device-related PIs. Barrier application has been shown to decrease pressure transmission and minimize prevalence and severity of PI across all ages.5-9 Many units have developed PI bundles. Elements of skin assessment, body and device repositioning, moisture control, support surfaces, and nutrition are commonly included. The author finds these elements to be important but not sufficient to prevent device-related PIs. A variety of foams, hydrocolloids, and contact layers can be incorporated into the bundle elements. Many contemporary studies have demonstrated success with barrier application.4-11 As COVID-19 numbers continue to increase, some units have implemented strategies of having a minimal number of providers care for patients who are infected. Adding barrier application and pressure offloading to such a policy is often employed in COVID-19 units.
Respiratory devices represent a special challenge because many noninvasive devices are produced based on adult shape and dimensions, not accounting for skin uniqueness and microclimate-induced changes in neonatal skin. Many manufacturers recommend the use of such devices without barriers, failing to recognize the effect they have on fragile pediatric skin. The author recommends barrier placement under most devices, as long as efficacy is not compromised. There is abundant literature supporting barrier placement as being beneficial in PI prevention.6-10
PI is and will continue to be one of the most challenging hospital-acquired conditions. The focus of caregivers needs to change from simply performing assessment scales (which do not decrease the prevalence of PI12) to understanding the physiology of PI development. If it is understood that deformation leads to cell injury, along with oxidative stress due to ischemia and compression of lymphatics, then practitioners will offload every device and tubing that may potentially deform underlying tissues and compress vessels. If the uniqueness of neonatal skin is understood, then adult recommendations will not be applied blindly, and practitioners will question the pitfalls that products have when used on neonatal skin. Manufacturers must recognize the effects of devices at the cellular level in neonatal and pediatric skin and the need to create and study devices based on neonatal and pediatric models. In addition, organizations must work on developing policies requiring increased attention to pediatric PIs.
The following examples show PIs that could have been prevented with appropriate barrier interphase.
Case 1. A stage 2 PI occurred from a noninvasive ventilation nasal mask in a 6-year-old patient admitted with respiratory failure secondary to pneumonia. Three (3) hours before the PI was discovered, the skin was assessed and the mask was repositioned. Mild redness was noted, yet foam was not placed. After the PI was discovered, nasal pressure interphase was changed, allowing the skin to heal (Fig. 1).
Case 2. A columellar injury was discovered in a preterm neonate after they were positioned prone and no barrier placed on the columellar, as per the cannula manufacturer’s suggestion (Fig. 2).
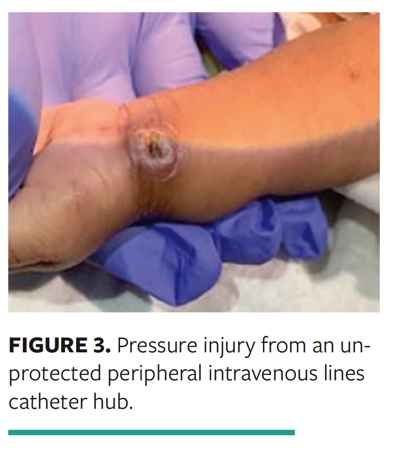
Case 3. A PI from an unprotected peripheral intravenous line catheter hub occurred in an agitated 1-month-old recovering from cardiac surgery (Fig. 3).
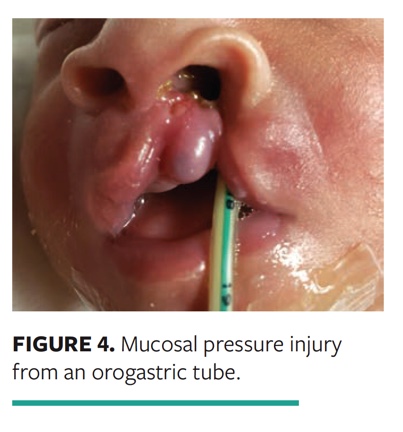
Case 4. A 2-week-old neonate had multiple comorbidities, including cleft lip and feeding insufficiency. Mucosal PI developed after friction between the alveolar ridge mucosa and orogastric tube while the child was prone (Fig. 4).
Case 5. A 16-month-old child was admitted with new-onset seizures. A stage 2 PI occurred in the form of an intact blister that was due to the pressure from an ill-fitting peripheral intravenous lines board (Fig. 5).
These examples represent the use of common daily medical devices. Understanding the pathophysiology may help clinicians to employ preventive measures, including various thin, offloading, and friction-minimizing barriers.
Dr. Boyar is director of Neonatal Wound Services, Cohen Children’s Medical Center of New York, New Hyde Park, and assistant professor of Pediatrics, Zucker School of Medicine, Hofstra/Northwell, Hempstead, NY. All photos provided are with the consent of the patients’ parents. This article was not subject to the Wound Management & Prevention peer-review process.
References
1. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059.
2. Baharestani M, Ratliff C. Pressure ulcers in neonates and children: an NPUAP white paper. Adv Skin Wound Care. 2007;20(4); 208, 210, 212, 214, 216, 218–220.
3. Bhattacharya S, Mishra R. Pressure ulcers: current understanding and newer modalities of treatment. Indian J Plast Surg. 2015;48(1):4–16.
4. Gefen A, Alves P, Ciprandi G, et al. Device-related pressure ulcers: SECURE prevention. J Wound Care. 2020;29(suppl 2A):S1–S52.
5. Gefen A. A new consensus on medical-related pressure ulcers. J Wound Care. 2019;28(6):315. doi:10.12968/jowc.2019.28.6.315
6. Schlüer A-B. Pressure ulcers in maturing skin - a clinical perspective. J Tissue Viabil. 2017;26:2–5.
7. Stellar J, Hasbani N, Kulik L, et al. Medical device-related pressure injuries in infants and children. J Wound Ostomy Continence Nurs. 2020;47(5):459–469.
8. Ambutas S, Staffileno BA, Fogg L. Reducing nasal pressure ulcers with an alternative taping device. Medsurg Nurs. 2014;23(2):96–100.
9. Black J, Alves P, Brindle CT, et al. Use of wound dressings to enhance prevention of pressure ulcers caused by medical devices. Int Wound J. 2013;12(3):322–327.
10. Kuo CY, Wooten CT, Tylor DA, Werkhaven JA, Huffman KF, Goudy SL. Prevention of pressure ulcers after pediatric tracheostomy using a Mepilex AG dressing. Laryngoscope. 2013;123(12):3201–3205.
11. Imbulana DI, Owen LS, Dawson JA, Bailey JL, Davis PG, Manley BJ. A randomized controlled trial of a barrier dressing to reduce nasal injury in preterm infants receiving binasal noninvasive respiratory support. J Pediatr. 2018;201:34–39.
12. More ZE, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev. 2014 Feb 5;(2):CD006471.