 It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics.
It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics.
Infectious Disease
All-natural Wart Remedy
Treatment of external anogenital warts can be frustrating and success limited. Standard therapy consists primarily of destructive methods (cryotherapy or blistering agents) that can be very painful. Topical therapy with imiquimod has also been less than optimal, often causing local irritation that can limit compliance. A recent meta-analysis examined the safety and efficacy of a topical green tea sinecatechin 15% ointment for the treatment of external anogenital warts. A total of 660 men and 587 women were included, with the primary outcome of complete clearance of warts. The topical sinecatechin 15% ointment demonstrated significantly higher likelihood of complete clearance of warts compared with controls and participants had very low rates of recurrence (10% to 12%). While there are no head-to-head studies, these data support the use of topical sinecatechins in the treatment of external warts. Furthermore, there may be a niche for this drug in patients who want an “all natural” therapy and/or cannot tolerate destructive methods.1
Genital Warts Treated with Recombinant Human Papillomavirus Vaccine
Lee et al report a case of condyloma accuminatum responding to a single course of recombinant quadrivalent human papillomavirus vaccine. The patient’s warts were increasing in size, despite treatment with topical imiquimod for 4 weeks. A mere 4 weeks after receiving the vaccine, the patient’s warts cleared. While there are no randomized controlled trials for using this vaccine with common warts, it may be an additional option to consider for warts resistant to standard therapies.2
Acne/Rosacea
Enhancing Patient Compliance with an Internet-based Survey
Teenagers have very poor adherence to topical acne therapy. Researchers at Wake Forest University are trying to find new ways to help motivate teens to use their medicine. A recent randomized controlled study examined the use of an Internet-based survey and contest to help improve adherence to daily application of topical benzoyl peroxide 5% gel. Patients enrolled in the intervention group received weekly emails containing a link to a Web-based survey. Patients were incentivized to fill out the survey with a chance to win an iPod Nano. Patients in the intervention group had significantly better mean adherence than the control group. Furthermore, the adherence of the intervention group did not significantly decrease over time. These groundbreaking results indicate that physicians can use technology to connect with teens and help increase adherence to treatment. Full application of this technology is still being tested.
 Psoriasis
Psoriasis
New Guidelines for Chronic Use of Methotrexate
Due to an association of liver toxicity and cirrhosis, physicians were advised to get liver biopsies on all patients who received a cumulative dose of 1 gram to 1.5 grams of methotrexate. New consensus guidelines have eliminated the compulsory liver biopsy after a set cumulative dose. Instead, it is recommended that physicians simply follow blood tests for liver function in low-risk patients.3
An Association between Treatment of Rheumatoid Arthritis and Psoriasis and a Decreased Risk of Diabetes
In a retrospective cohort study by Solomon et al, the authors found that among patients with either psoriasis or rheumatoid arthritis, treatment with hydroxychloroquine or tumor necrosis factor (TNF)–inhibiting agents was associated with a decreased incidence of diabetes.4 The cohort consisted of 13,905 patients who were followed up for a mean of 5.8 months after the first prescription for a disease-modifying antirheumatic drug (DMARD). Patients were assessed for a new diagnosis of diabetes mellitus. After accounting for possible confounding factors such as age, sex and other clinical variables, the hazard ratios for incident diabetes were 0.54 (95% confidence interval [CI], 0.36-0.80) for patients treated with hydroxychloroquine, 0.62 (95% CI, 0.42-0.91) for those treated with TNF inhibitors and 0.77 (95% CI, 0.53-1.13) for those treated with methotrexate, compared with patients treated with other anti-inflammatory medications.
New Gene Identified in Pustular Psoriasis
Scientists are constantly searching for genes that are implicated in the pathogenesis of psoriasis. Recently, Marrakchi et al found a single gene mutation that appears to be largely responsible for the manifestation of pustular psoriasis.5 The researchers described a homozygous mutation in the gene that encodes interleukin-36–receptor antagonist (IL-36Ra). This mutated receptor antagonist is very poor at inhibiting pro-inflammatory pathways and affected keratinocytes produce more IL-8 and other pro-inflammatory mediators as a downstream result. In an age of selective immunotherapy, this single gene mutation represents another potential target for intervention.
 Photoprotection
Photoprotection
Prospective Evidence for Reduced Risk of Melanoma with Sunscreen
In a recent prospective randomized controlled trial by Green et al, the authors examined the impact of daily sunscreen use compared with discretionary sunscreen use over a 14-year period on the development of melanoma.6,7 A total of 1,621 randomly selected residents of Nambour, Queensland, Australia age 25 to 75 years were enrolled in 1992. Participants were followed through 2006, using questionnaires, pathology laboratory reports and the Queensland Cancer Registry to ascertain primary melanoma occurrence. During the 10-year follow up period after trial cessation, 11 new primary melanomas had been identified in the daily sunscreen group and 22 had been identified in the discretionary group. This represented a reduction of the observed rate of any melanoma in those randomly assigned to daily sunscreen use (hazard ratio [HR], 0.50; 95% CI, 0.24 to 1.02; P = .051). When examining only invasive melanomas, this reduction was even more substantial (n = 3 in active vs. 11 in control; HR, 0.27; 95% CI, 0.08 to 0.97). This study provides additional good quality data to support the use of sunscreens as a method to reduce one’s risk of melanoma.
Controversies in Sunscreen Safety
While sunscreens have been a primary method to help prevent skin cancer, there have been a few articles questioning their overall safety. In particular, some authors have purported the potential for systemic toxicity by oxybenzone, retinyl palmitate and nano-sized particles of zinc oxide and titanium dioxide. In a recent article by Burnett and Wang, the data was reviewed critically to help address these concerns.8 The authors concluded that much of the data supporting the potential for systemic toxicity was extrapolated from in vitro and/or animal studies. However, data from human studies did not demonstrate ill effects from any of the sunscreen ingredients in question. Furthermore, the concern for systemic toxicity is largely based on the systemic absorption profiles of the topically applied compounds. However, similar systemic levels achieved by animal studies (>1500mg/kg/d of oxybenzone) are essentially unattainable with real-life topical application.9,10
Vitamin D and Sunscreen Use
The importance of vitamin D has been in the spotlight for the past few years and there has beensome evidence that sunscreens may reduce vitamin D levels. This has led a few physicians and non-medical readers to be discouraged by their regular use. However, when looking at the plethora of data, sunscreen use appears to have little to no impact on clinically relevant vitamin D levels.8 Furthermore, if patients are ever concerned, they can always get vitamin D by oral supplementation while still getting the sun-protective benefits of sunscreen.
Cutaneous Malignancy
Identifying Populations at Greater Risk for Melanoma
 Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories.
Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories.
Melanoma in the Transplant Patient
A recent retrospective review examined overall survival among patients with melanoma diagnosed after organ transplantation compared with a national sample of patients with malignant melanoma.12 In the review, malignant melanoma was diagnosed in 638 patients after transplantation; Breslow thickness was available for 123 of these patients. The 3-year overall survival rates for patients stratified by Breslow thickness (≤0.75, 0.76-1.50, 1.51-3.00 and >3.00 mm) were 88.2%, 80.8%, 51.2% and 55.3%, respectively. Interestingly, survival rates for thicker melanomas (Breslow 1.5 to 3 mm) were significantly poorer in the transplant recipients compared with the expected survival rates derived from the Surveillance, Epidemiology and End Results Program.
New Hope in the Battle Against Melanoma
 Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas.
Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas.
Vemurafenib is an orally administered small molecular kinase inhibitor. It is indicated for the treatment of patients with unresectable or metastatic melanoma with a specific BRAF V600E mutation.
A randomized, open-label, controlled, multicenter, phase III study (BRIM3) compared vemurafenib to dacarbazine, a standard-of-care chemotherapy. The study examined 675 patients with previously untreated BRAF V600E mutation-positive, unresectable or metastatic melanoma.13 The endpoints were overall survival (OS) and investigator-assessed progression-free survival (PFS). At 6 months, overall survival was 84% in the vemurafenib group and 64% in the dacarbazine group. The results indicated that the risk of death was reduced by 56% for people who received vemurafenib compared to those who received chemotherapy (hazard ratio [HR] = 0.44, p<0.0001). Patients who received vemurafenib also had a better PFS, with a median PFS of 5.3 months compared to 1.6 months for those who received chemotherapy. This translated to a 74% reduced risk of the disease getting worse or the patient dying compared to those who received chemotherapy (HR=0.26, p<0.0001). When examining those who experienced tumor shrinkage (confirmed by investigator-assessed response rate), people who received vemurafenib had a 47.4% partial response rate compared to a 5.5% partial response rate for those who received chemotherapy (p<0.0001). An additional 1% receiving vemurafenib had a complete response. No one receiving standard chemotherapy had a complete response. These results provide an additional unprecedented hope in the battle against melanoma.
Other new drugs have also arisen in the fight against melanoma. Ipilimumab (Yervoy), a monoclonal antibody targeting the ligand CTLA-4, was approved the by FDA this year for patients with advanced melanoma as a first- and second-line treatment.14 Robert et al reported a significant survival benefit of using ipilimumab in combination with dacarbazine compared with dacarbazine alone for first-line treatment of metastatic melanoma.15
Another recently approved adjuvant treatment, pegylated interferon-α2b, demonstrated a sustained impact on relapse-free survival in patients with lymph node-positive melanoma. Looking ahead at other monoclonal antibodies, programmed death-1 (PD-1) targets T-cell ligands and has shown promise in phase I trials for melanoma.
 Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma.
Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma.
Biologics
Rituximab in the Treatment of Chronic Bullous Disorders
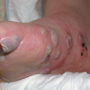 Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.
Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.
Similarly, another case series by Le Roux-Villet et al reported 25 patients with refractory mucous membrane pemphigoid (MMP) who were treated with rituximab.24 All patients received one or two cycles of rituximab weekly for 4 weeks. Seventeen of the 25 patients achieved complete response with one treatment, with a median response time of 12 weeks after first treatment. Another five patients had complete response after a second round of treatment, yielding a total 88% complete response rate. No immediate serious adverse events were observed. However, three patients developed serious infections 4 months after rituximab therapy, two of whom died from their infection. While rituximab appears to be efficacious for MMP, further investigation is needed to ascertain the optimum dosing regimen and safety.
Summary
From new therapies for melanoma to newly-identified genes in psoriasis, it has been an exciting year in dermatology. Thanks to all of the hard work from researchers and dermatologists around the globe, our specialty continues to grow strong. We look forward to what the next year has to offer!
Dr. Yentzer is with the Department of Dermatology, Wake Forest Baptist Health in Winston-Salem, NC.
Disclosure: The author has no conflicts of interest to disclose.
 It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics.
It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics.
Infectious Disease
All-natural Wart Remedy
Treatment of external anogenital warts can be frustrating and success limited. Standard therapy consists primarily of destructive methods (cryotherapy or blistering agents) that can be very painful. Topical therapy with imiquimod has also been less than optimal, often causing local irritation that can limit compliance. A recent meta-analysis examined the safety and efficacy of a topical green tea sinecatechin 15% ointment for the treatment of external anogenital warts. A total of 660 men and 587 women were included, with the primary outcome of complete clearance of warts. The topical sinecatechin 15% ointment demonstrated significantly higher likelihood of complete clearance of warts compared with controls and participants had very low rates of recurrence (10% to 12%). While there are no head-to-head studies, these data support the use of topical sinecatechins in the treatment of external warts. Furthermore, there may be a niche for this drug in patients who want an “all natural” therapy and/or cannot tolerate destructive methods.1
Genital Warts Treated with Recombinant Human Papillomavirus Vaccine
Lee et al report a case of condyloma accuminatum responding to a single course of recombinant quadrivalent human papillomavirus vaccine. The patient’s warts were increasing in size, despite treatment with topical imiquimod for 4 weeks. A mere 4 weeks after receiving the vaccine, the patient’s warts cleared. While there are no randomized controlled trials for using this vaccine with common warts, it may be an additional option to consider for warts resistant to standard therapies.2
Acne/Rosacea
Enhancing Patient Compliance with an Internet-based Survey
Teenagers have very poor adherence to topical acne therapy. Researchers at Wake Forest University are trying to find new ways to help motivate teens to use their medicine. A recent randomized controlled study examined the use of an Internet-based survey and contest to help improve adherence to daily application of topical benzoyl peroxide 5% gel. Patients enrolled in the intervention group received weekly emails containing a link to a Web-based survey. Patients were incentivized to fill out the survey with a chance to win an iPod Nano. Patients in the intervention group had significantly better mean adherence than the control group. Furthermore, the adherence of the intervention group did not significantly decrease over time. These groundbreaking results indicate that physicians can use technology to connect with teens and help increase adherence to treatment. Full application of this technology is still being tested.
 Psoriasis
Psoriasis
New Guidelines for Chronic Use of Methotrexate
Due to an association of liver toxicity and cirrhosis, physicians were advised to get liver biopsies on all patients who received a cumulative dose of 1 gram to 1.5 grams of methotrexate. New consensus guidelines have eliminated the compulsory liver biopsy after a set cumulative dose. Instead, it is recommended that physicians simply follow blood tests for liver function in low-risk patients.3
An Association between Treatment of Rheumatoid Arthritis and Psoriasis and a Decreased Risk of Diabetes
In a retrospective cohort study by Solomon et al, the authors found that among patients with either psoriasis or rheumatoid arthritis, treatment with hydroxychloroquine or tumor necrosis factor (TNF)–inhibiting agents was associated with a decreased incidence of diabetes.4 The cohort consisted of 13,905 patients who were followed up for a mean of 5.8 months after the first prescription for a disease-modifying antirheumatic drug (DMARD). Patients were assessed for a new diagnosis of diabetes mellitus. After accounting for possible confounding factors such as age, sex and other clinical variables, the hazard ratios for incident diabetes were 0.54 (95% confidence interval [CI], 0.36-0.80) for patients treated with hydroxychloroquine, 0.62 (95% CI, 0.42-0.91) for those treated with TNF inhibitors and 0.77 (95% CI, 0.53-1.13) for those treated with methotrexate, compared with patients treated with other anti-inflammatory medications.
New Gene Identified in Pustular Psoriasis
Scientists are constantly searching for genes that are implicated in the pathogenesis of psoriasis. Recently, Marrakchi et al found a single gene mutation that appears to be largely responsible for the manifestation of pustular psoriasis.5 The researchers described a homozygous mutation in the gene that encodes interleukin-36–receptor antagonist (IL-36Ra). This mutated receptor antagonist is very poor at inhibiting pro-inflammatory pathways and affected keratinocytes produce more IL-8 and other pro-inflammatory mediators as a downstream result. In an age of selective immunotherapy, this single gene mutation represents another potential target for intervention.
 Photoprotection
Photoprotection
Prospective Evidence for Reduced Risk of Melanoma with Sunscreen
In a recent prospective randomized controlled trial by Green et al, the authors examined the impact of daily sunscreen use compared with discretionary sunscreen use over a 14-year period on the development of melanoma.6,7 A total of 1,621 randomly selected residents of Nambour, Queensland, Australia age 25 to 75 years were enrolled in 1992. Participants were followed through 2006, using questionnaires, pathology laboratory reports and the Queensland Cancer Registry to ascertain primary melanoma occurrence. During the 10-year follow up period after trial cessation, 11 new primary melanomas had been identified in the daily sunscreen group and 22 had been identified in the discretionary group. This represented a reduction of the observed rate of any melanoma in those randomly assigned to daily sunscreen use (hazard ratio [HR], 0.50; 95% CI, 0.24 to 1.02; P = .051). When examining only invasive melanomas, this reduction was even more substantial (n = 3 in active vs. 11 in control; HR, 0.27; 95% CI, 0.08 to 0.97). This study provides additional good quality data to support the use of sunscreens as a method to reduce one’s risk of melanoma.
Controversies in Sunscreen Safety
While sunscreens have been a primary method to help prevent skin cancer, there have been a few articles questioning their overall safety. In particular, some authors have purported the potential for systemic toxicity by oxybenzone, retinyl palmitate and nano-sized particles of zinc oxide and titanium dioxide. In a recent article by Burnett and Wang, the data was reviewed critically to help address these concerns.8 The authors concluded that much of the data supporting the potential for systemic toxicity was extrapolated from in vitro and/or animal studies. However, data from human studies did not demonstrate ill effects from any of the sunscreen ingredients in question. Furthermore, the concern for systemic toxicity is largely based on the systemic absorption profiles of the topically applied compounds. However, similar systemic levels achieved by animal studies (>1500mg/kg/d of oxybenzone) are essentially unattainable with real-life topical application.9,10
Vitamin D and Sunscreen Use
The importance of vitamin D has been in the spotlight for the past few years and there has beensome evidence that sunscreens may reduce vitamin D levels. This has led a few physicians and non-medical readers to be discouraged by their regular use. However, when looking at the plethora of data, sunscreen use appears to have little to no impact on clinically relevant vitamin D levels.8 Furthermore, if patients are ever concerned, they can always get vitamin D by oral supplementation while still getting the sun-protective benefits of sunscreen.
Cutaneous Malignancy
Identifying Populations at Greater Risk for Melanoma
 Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories.
Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories.
Melanoma in the Transplant Patient
A recent retrospective review examined overall survival among patients with melanoma diagnosed after organ transplantation compared with a national sample of patients with malignant melanoma.12 In the review, malignant melanoma was diagnosed in 638 patients after transplantation; Breslow thickness was available for 123 of these patients. The 3-year overall survival rates for patients stratified by Breslow thickness (≤0.75, 0.76-1.50, 1.51-3.00 and >3.00 mm) were 88.2%, 80.8%, 51.2% and 55.3%, respectively. Interestingly, survival rates for thicker melanomas (Breslow 1.5 to 3 mm) were significantly poorer in the transplant recipients compared with the expected survival rates derived from the Surveillance, Epidemiology and End Results Program.
New Hope in the Battle Against Melanoma
 Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas.
Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas.
Vemurafenib is an orally administered small molecular kinase inhibitor. It is indicated for the treatment of patients with unresectable or metastatic melanoma with a specific BRAF V600E mutation.
A randomized, open-label, controlled, multicenter, phase III study (BRIM3) compared vemurafenib to dacarbazine, a standard-of-care chemotherapy. The study examined 675 patients with previously untreated BRAF V600E mutation-positive, unresectable or metastatic melanoma.13 The endpoints were overall survival (OS) and investigator-assessed progression-free survival (PFS). At 6 months, overall survival was 84% in the vemurafenib group and 64% in the dacarbazine group. The results indicated that the risk of death was reduced by 56% for people who received vemurafenib compared to those who received chemotherapy (hazard ratio [HR] = 0.44, p<0.0001). Patients who received vemurafenib also had a better PFS, with a median PFS of 5.3 months compared to 1.6 months for those who received chemotherapy. This translated to a 74% reduced risk of the disease getting worse or the patient dying compared to those who received chemotherapy (HR=0.26, p<0.0001). When examining those who experienced tumor shrinkage (confirmed by investigator-assessed response rate), people who received vemurafenib had a 47.4% partial response rate compared to a 5.5% partial response rate for those who received chemotherapy (p<0.0001). An additional 1% receiving vemurafenib had a complete response. No one receiving standard chemotherapy had a complete response. These results provide an additional unprecedented hope in the battle against melanoma.
Other new drugs have also arisen in the fight against melanoma. Ipilimumab (Yervoy), a monoclonal antibody targeting the ligand CTLA-4, was approved the by FDA this year for patients with advanced melanoma as a first- and second-line treatment.14 Robert et al reported a significant survival benefit of using ipilimumab in combination with dacarbazine compared with dacarbazine alone for first-line treatment of metastatic melanoma.15
Another recently approved adjuvant treatment, pegylated interferon-α2b, demonstrated a sustained impact on relapse-free survival in patients with lymph node-positive melanoma. Looking ahead at other monoclonal antibodies, programmed death-1 (PD-1) targets T-cell ligands and has shown promise in phase I trials for melanoma.
 Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma.
Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma.
Biologics
Rituximab in the Treatment of Chronic Bullous Disorders
 Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.
Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.
Similarly, another case series by Le Roux-Villet et al reported 25 patients with refractory mucous membrane pemphigoid (MMP) who were treated with rituximab.24 All patients received one or two cycles of rituximab weekly for 4 weeks. Seventeen of the 25 patients achieved complete response with one treatment, with a median response time of 12 weeks after first treatment. Another five patients had complete response after a second round of treatment, yielding a total 88% complete response rate. No immediate serious adverse events were observed. However, three patients developed serious infections 4 months after rituximab therapy, two of whom died from their infection. While rituximab appears to be efficacious for MMP, further investigation is needed to ascertain the optimum dosing regimen and safety.
Summary
From new therapies for melanoma to newly-identified genes in psoriasis, it has been an exciting year in dermatology. Thanks to all of the hard work from researchers and dermatologists around the globe, our specialty continues to grow strong. We look forward to what the next year has to offer!
Dr. Yentzer is with the Department of Dermatology, Wake Forest Baptist Health in Winston-Salem, NC.
Disclosure: The author has no conflicts of interest to disclose.
 It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics.
It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics.
Infectious Disease
All-natural Wart Remedy
Treatment of external anogenital warts can be frustrating and success limited. Standard therapy consists primarily of destructive methods (cryotherapy or blistering agents) that can be very painful. Topical therapy with imiquimod has also been less than optimal, often causing local irritation that can limit compliance. A recent meta-analysis examined the safety and efficacy of a topical green tea sinecatechin 15% ointment for the treatment of external anogenital warts. A total of 660 men and 587 women were included, with the primary outcome of complete clearance of warts. The topical sinecatechin 15% ointment demonstrated significantly higher likelihood of complete clearance of warts compared with controls and participants had very low rates of recurrence (10% to 12%). While there are no head-to-head studies, these data support the use of topical sinecatechins in the treatment of external warts. Furthermore, there may be a niche for this drug in patients who want an “all natural” therapy and/or cannot tolerate destructive methods.1
Genital Warts Treated with Recombinant Human Papillomavirus Vaccine
Lee et al report a case of condyloma accuminatum responding to a single course of recombinant quadrivalent human papillomavirus vaccine. The patient’s warts were increasing in size, despite treatment with topical imiquimod for 4 weeks. A mere 4 weeks after receiving the vaccine, the patient’s warts cleared. While there are no randomized controlled trials for using this vaccine with common warts, it may be an additional option to consider for warts resistant to standard therapies.2
Acne/Rosacea
Enhancing Patient Compliance with an Internet-based Survey
Teenagers have very poor adherence to topical acne therapy. Researchers at Wake Forest University are trying to find new ways to help motivate teens to use their medicine. A recent randomized controlled study examined the use of an Internet-based survey and contest to help improve adherence to daily application of topical benzoyl peroxide 5% gel. Patients enrolled in the intervention group received weekly emails containing a link to a Web-based survey. Patients were incentivized to fill out the survey with a chance to win an iPod Nano. Patients in the intervention group had significantly better mean adherence than the control group. Furthermore, the adherence of the intervention group did not significantly decrease over time. These groundbreaking results indicate that physicians can use technology to connect with teens and help increase adherence to treatment. Full application of this technology is still being tested.
 Psoriasis
Psoriasis
New Guidelines for Chronic Use of Methotrexate
Due to an association of liver toxicity and cirrhosis, physicians were advised to get liver biopsies on all patients who received a cumulative dose of 1 gram to 1.5 grams of methotrexate. New consensus guidelines have eliminated the compulsory liver biopsy after a set cumulative dose. Instead, it is recommended that physicians simply follow blood tests for liver function in low-risk patients.3
An Association between Treatment of Rheumatoid Arthritis and Psoriasis and a Decreased Risk of Diabetes
In a retrospective cohort study by Solomon et al, the authors found that among patients with either psoriasis or rheumatoid arthritis, treatment with hydroxychloroquine or tumor necrosis factor (TNF)–inhibiting agents was associated with a decreased incidence of diabetes.4 The cohort consisted of 13,905 patients who were followed up for a mean of 5.8 months after the first prescription for a disease-modifying antirheumatic drug (DMARD). Patients were assessed for a new diagnosis of diabetes mellitus. After accounting for possible confounding factors such as age, sex and other clinical variables, the hazard ratios for incident diabetes were 0.54 (95% confidence interval [CI], 0.36-0.80) for patients treated with hydroxychloroquine, 0.62 (95% CI, 0.42-0.91) for those treated with TNF inhibitors and 0.77 (95% CI, 0.53-1.13) for those treated with methotrexate, compared with patients treated with other anti-inflammatory medications.
New Gene Identified in Pustular Psoriasis
Scientists are constantly searching for genes that are implicated in the pathogenesis of psoriasis. Recently, Marrakchi et al found a single gene mutation that appears to be largely responsible for the manifestation of pustular psoriasis.5 The researchers described a homozygous mutation in the gene that encodes interleukin-36–receptor antagonist (IL-36Ra). This mutated receptor antagonist is very poor at inhibiting pro-inflammatory pathways and affected keratinocytes produce more IL-8 and other pro-inflammatory mediators as a downstream result. In an age of selective immunotherapy, this single gene mutation represents another potential target for intervention.
 Photoprotection
Photoprotection
Prospective Evidence for Reduced Risk of Melanoma with Sunscreen
In a recent prospective randomized controlled trial by Green et al, the authors examined the impact of daily sunscreen use compared with discretionary sunscreen use over a 14-year period on the development of melanoma.6,7 A total of 1,621 randomly selected residents of Nambour, Queensland, Australia age 25 to 75 years were enrolled in 1992. Participants were followed through 2006, using questionnaires, pathology laboratory reports and the Queensland Cancer Registry to ascertain primary melanoma occurrence. During the 10-year follow up period after trial cessation, 11 new primary melanomas had been identified in the daily sunscreen group and 22 had been identified in the discretionary group. This represented a reduction of the observed rate of any melanoma in those randomly assigned to daily sunscreen use (hazard ratio [HR], 0.50; 95% CI, 0.24 to 1.02; P = .051). When examining only invasive melanomas, this reduction was even more substantial (n = 3 in active vs. 11 in control; HR, 0.27; 95% CI, 0.08 to 0.97). This study provides additional good quality data to support the use of sunscreens as a method to reduce one’s risk of melanoma.
Controversies in Sunscreen Safety
While sunscreens have been a primary method to help prevent skin cancer, there have been a few articles questioning their overall safety. In particular, some authors have purported the potential for systemic toxicity by oxybenzone, retinyl palmitate and nano-sized particles of zinc oxide and titanium dioxide. In a recent article by Burnett and Wang, the data was reviewed critically to help address these concerns.8 The authors concluded that much of the data supporting the potential for systemic toxicity was extrapolated from in vitro and/or animal studies. However, data from human studies did not demonstrate ill effects from any of the sunscreen ingredients in question. Furthermore, the concern for systemic toxicity is largely based on the systemic absorption profiles of the topically applied compounds. However, similar systemic levels achieved by animal studies (>1500mg/kg/d of oxybenzone) are essentially unattainable with real-life topical application.9,10
Vitamin D and Sunscreen Use
The importance of vitamin D has been in the spotlight for the past few years and there has beensome evidence that sunscreens may reduce vitamin D levels. This has led a few physicians and non-medical readers to be discouraged by their regular use. However, when looking at the plethora of data, sunscreen use appears to have little to no impact on clinically relevant vitamin D levels.8 Furthermore, if patients are ever concerned, they can always get vitamin D by oral supplementation while still getting the sun-protective benefits of sunscreen.
Cutaneous Malignancy
Identifying Populations at Greater Risk for Melanoma
 Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories.
Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories.
Melanoma in the Transplant Patient
A recent retrospective review examined overall survival among patients with melanoma diagnosed after organ transplantation compared with a national sample of patients with malignant melanoma.12 In the review, malignant melanoma was diagnosed in 638 patients after transplantation; Breslow thickness was available for 123 of these patients. The 3-year overall survival rates for patients stratified by Breslow thickness (≤0.75, 0.76-1.50, 1.51-3.00 and >3.00 mm) were 88.2%, 80.8%, 51.2% and 55.3%, respectively. Interestingly, survival rates for thicker melanomas (Breslow 1.5 to 3 mm) were significantly poorer in the transplant recipients compared with the expected survival rates derived from the Surveillance, Epidemiology and End Results Program.
New Hope in the Battle Against Melanoma
 Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas.
Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas.
Vemurafenib is an orally administered small molecular kinase inhibitor. It is indicated for the treatment of patients with unresectable or metastatic melanoma with a specific BRAF V600E mutation.
A randomized, open-label, controlled, multicenter, phase III study (BRIM3) compared vemurafenib to dacarbazine, a standard-of-care chemotherapy. The study examined 675 patients with previously untreated BRAF V600E mutation-positive, unresectable or metastatic melanoma.13 The endpoints were overall survival (OS) and investigator-assessed progression-free survival (PFS). At 6 months, overall survival was 84% in the vemurafenib group and 64% in the dacarbazine group. The results indicated that the risk of death was reduced by 56% for people who received vemurafenib compared to those who received chemotherapy (hazard ratio [HR] = 0.44, p<0.0001). Patients who received vemurafenib also had a better PFS, with a median PFS of 5.3 months compared to 1.6 months for those who received chemotherapy. This translated to a 74% reduced risk of the disease getting worse or the patient dying compared to those who received chemotherapy (HR=0.26, p<0.0001). When examining those who experienced tumor shrinkage (confirmed by investigator-assessed response rate), people who received vemurafenib had a 47.4% partial response rate compared to a 5.5% partial response rate for those who received chemotherapy (p<0.0001). An additional 1% receiving vemurafenib had a complete response. No one receiving standard chemotherapy had a complete response. These results provide an additional unprecedented hope in the battle against melanoma.
Other new drugs have also arisen in the fight against melanoma. Ipilimumab (Yervoy), a monoclonal antibody targeting the ligand CTLA-4, was approved the by FDA this year for patients with advanced melanoma as a first- and second-line treatment.14 Robert et al reported a significant survival benefit of using ipilimumab in combination with dacarbazine compared with dacarbazine alone for first-line treatment of metastatic melanoma.15
Another recently approved adjuvant treatment, pegylated interferon-α2b, demonstrated a sustained impact on relapse-free survival in patients with lymph node-positive melanoma. Looking ahead at other monoclonal antibodies, programmed death-1 (PD-1) targets T-cell ligands and has shown promise in phase I trials for melanoma.
 Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma.
Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma.
Biologics
Rituximab in the Treatment of Chronic Bullous Disorders
 Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.
Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.
Similarly, another case series by Le Roux-Villet et al reported 25 patients with refractory mucous membrane pemphigoid (MMP) who were treated with rituximab.24 All patients received one or two cycles of rituximab weekly for 4 weeks. Seventeen of the 25 patients achieved complete response with one treatment, with a median response time of 12 weeks after first treatment. Another five patients had complete response after a second round of treatment, yielding a total 88% complete response rate. No immediate serious adverse events were observed. However, three patients developed serious infections 4 months after rituximab therapy, two of whom died from their infection. While rituximab appears to be efficacious for MMP, further investigation is needed to ascertain the optimum dosing regimen and safety.
Summary
From new therapies for melanoma to newly-identified genes in psoriasis, it has been an exciting year in dermatology. Thanks to all of the hard work from researchers and dermatologists around the globe, our specialty continues to grow strong. We look forward to what the next year has to offer!
Dr. Yentzer is with the Department of Dermatology, Wake Forest Baptist Health in Winston-Salem, NC.
Disclosure: The author has no conflicts of interest to disclose.
 It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics.
It has been another exciting year in the field of dermatology. This year-in-review will highlight some of the clinically relevant discoveries and techniques of 2011. Areas of dermatology that this review will cover include infectious disease, acne, psoriasis, cutaneous malignancy, photoprotection, pediatric dermatology, contact dermatitis, biological immunomodulators and cosmetics. Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories.
Over the past 30 years, melanoma among young white females has more than doubled. A recent longitudinal study of 3,800 white women age 15 to 39 in California demonstrated that individuals living in an area of high socioeconomic status (SES) and high UV radiation had a greater increase in rates of melanoma than areas of lower SES.11 While melanoma rates increased over time for all SES categories, those living in neighborhoods with the highest SES categories had 80% higher rates of melanoma than those in neighborhoods in the lowest categories. Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas.
Melanoma is one of the most feared skin cancers, as therapeutic options for metastatic disease are limited. The FDA recently approved vemurafenib (Zelboraf) for the treatment of certain inoperable or metastatic melanomas. Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma.
Also, in November, the FDA approved the pre-market approval (PMA) application for MelaFind. According to MELA Sciences, the manufacturer, MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma. The device is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy but should not be used to confirm a clinical diagnosis of melanoma. Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.
Rituximab is emerging as an effective therapy for blistering diseases such as pemphigus vulgaris and bullous pemphigoid. A case series by Kasperkiewicz et al reviewed treatment of 17 patients with a variety of refractory autoimmune blistering dermatoses, including pemphigus vulgaris (PV), n = 8; pemphigus foliaceus (PF), n = 2; bullous pemphigoid (BP), n = 2; and mucous membrane pemphigoid, n = 5.23 Of these 17 patients, eight showed complete remission off therapy (six PV, one PF and one MMP). Treatment length and regimens varied and many of the patients were also continued on other immunosuppressive therapy during the treatment. More than half of the patients experienced relapses after the initial course but responded to retreatment.

































