Dr. Graham was the former head of dermatopathology at the Armed Forces Institute of Pathology and the former chair of dermatology at UC Irvine in Irvine, CA. He has made available his personal library of kodachromes, slides and lectures collected throughout his career with a generous donation to the Wake Forest University Baptist Medical Center Library. Dr. Graham’s lectures and personal images in dermatomycology will be highlighted in this series to refresh the practicing clinician on deep cutaneous mycotic disease.
Rhinosporidiosis is a chronic, granulomatous mucous membrane infection presenting most commonly as a polyploid mass of the nasal, oral, ocular or genital mucosa.1 The taxonomy of the etiologic agent responsible for the disease has been long debated. First described in 1896 as a sporozoan by Guillermo Seeber, it was believed for several years to be a fungus and given the name Rhinosporidium seeberi by J.H. Ashworth in 1923.2,3 Recently, sequence analysis of an 18S small ribosomal DNA subunit has again redirected the debate. Due to this finding, the infectious agent is now grouped among a clade of fish parasites known as Mesomycetozoea.4 While its taxonomic position still remains unclear, this latest theory appears to be the most favored by researchers. Cutaneous involvement is rare and usually occurs after dissemination, arising from autoinoculation, direct implantation or hematogenous spread.2,5 Infection can cause significant morbidity in the form of functional obstruction, cosmetic injury and recurrence. Although the occurrence of systemic disease is rare, it may result in wasting, fever or even death secondary to lesions of the liver, kidneys, lungs, bone or spleen.1 To reduce morbidity and relieve any chance of mortality due to this disease, prompt recognition and adequate treatment are necessary.2
Epidemiology and Pathogenesis
R. seeberi, long believed to be of fungal origin, is now theorized to be an aquatic protistan parasite of the Mesomycetozoea class. It is thought to be contracted via contaminated water, dust or fomites.6 Although reported in Europe, Africa and the Americas, rhinosporidiosis is endemic in India and Sri Lanka.1,4,6 Migration from Asian countries to the West has led to a prevalence in the tropics.1 Bathing in stagnant or contaminated fresh water, a common practice in the Indian subcontinent, appears to be a risk factor.5-7 Although women tend to present more frequently with ocular involvement, a higher incidence of the most common presentation, nasal and nasopharyngeal infection, is seen in males age 20 to 40 years.3,6
A traumatized epithelium allows for a direct inoculation of the infectious agent, which produces endosporulating cells in the infected host and forms thick-walled sporangia within host subepithelium.1 Mature forms also contain smaller endospores (Figure 1).1,8 Following infection, dissemination may occur via autoinoculation or hematogenous spread. The former, usually the product of scratching, leads to satellite lesions adjacent to the primary site, while the latter may initiate spread to anatomically distant sites or fulminant systemic involvement.9
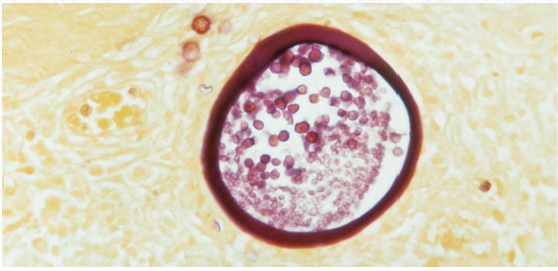
Figure 1. Rhinosporidiosis sporangia with endospores. Source: Graham Library of Digital Images, Wake Forest University Department
of Dermatology © 2009 Wake Forest University Dermatology
Clinical Presentation
Cutaneous lesions begin as tiny papules, and slowly evolve into large warty, poly-poidal masses.10 Nasal involvement can lead to nasal obstruction and bleeding, as masses can become quite large and tend to be very friable and vascular. The pedunculated or sessile polyp will have a purple-red surface studded with small white dots representing the sporangium within the epithelium. This pattern gives the lesion its characteristic strawberry-like appearance.1,3
Histopathology
A biopsy reveals chronic lymphocytic infiltrate along with the presence of plasma cells and foreign body giant cells (Figure 2).2
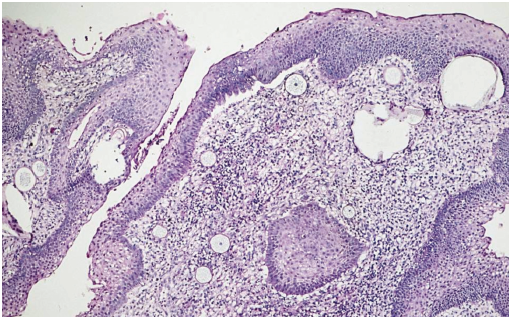
Figure 2. Rhinosporidiosis biopsy showing inflammatory infiltrate and characteristic sporangia. Source: Graham Library of Digital Images, Wake Forest University Department of Dermatology © 2009 Wake Forest University Dermatology
Within the subepithelial connective tissue of the host, a large number of thick, chitinous-walled, globular sporangia may be seen in various sizes and stages of maturation (10->450 µm). Many of these sporangia contain numerous endospores (6-12 µm), but those lacking maturity or having collapsed do not (Figure 3).2,3
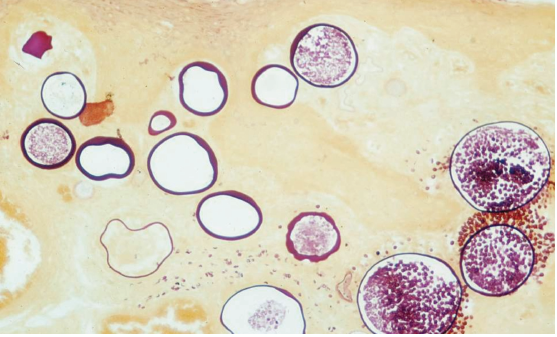
Figure 3. Mix of mature (containing endospores), immature and collapsed sporangia within host subepithelium. Source: Graham
Library of Digital Images, Wake Forest University Department of Dermatology © 2009 Wake Forest University Dermatology
Differential Diagnosis
Because cutaneous rhinosporidiosis is so rare, a high degree of clinical suspicion and histopathological evaluation is required to differentiate the illness from other mimickers. It may be confused with a long list of diseases presenting with similar-appearing papules or masses including verruca vulgaris, coccidioidomycosis, bacillary angiomatosis, pyogenic granuloma, verrucous tuberculosis, donovanosis and ecthyma (Table).3,6,7,9 Of these, rhinosporidiosis is most commonly mistaken for coccidioidomycosis. However, morphology, patient history and histological presentation draw a clear distinction between the two. While the pair share a histological similarity in that they both possess sporangia, the spores of coccidioidomycosis are much smaller (<60 µm in diameter).3
Diagnostic Tests
R seeberi growth in culture is ineffective, and therefore, other means of identification are necessary, including fine-needle aspiration, imprint cytology and skin biopsy.5 Following aspiration cytology, examining the smear with Papanicolaou staining or 10% potassium hydroxide may allow for identification of the organism in different stages of maturation. Giemsa stained imprint smears may also prove to be an effective modality.2,9 However, histopathological examination remains the diagnostic method of choice.2,5,9 Detection of the characteristic bilamellar, thick-walled sporangia containing endospores may be accomplished using haematoxylin and eosin (H&E) stained sections. For better visualization and enhanced histopathological assessment, special stains such as Gomori methenamine-silver stain, periodic acid-Schiff (PAS) stain and mucicarmine stain should be used.4
Treatment
In general, the prognosis of cutaneous rhinosporidiosis is unfavorable due to its unrelenting behavior and tendency to recur.2 Spontaneous regression rarely occurs, and extension or dissemination of lesions is a possibility, encouraging prompt treatment. The gold standard of rhinosporidiosis therapy is surgical excision with electrodesiccation.1,9,11 However, surgery brings with it the risk of hematological dissemination, hemorrhage and nasal septal perforation, and even with complete excision, lesions still have a propensity to recur.1,9 Adjuvant medical therapies, including trimethoprim/sulfadiazine, sodium stibogluconate and antifungals (such as griseofulvin and amphotericin B), have been attempted with limited success.1 Currently, dapsone is the only effective drug used as a surgical adjunct. As evidenced by light and electron microscopic studies, dapsone acts via arrest of sporangial maturation and through the promotion of stromal fibrosis. It also prevents any post-surgery colonization or adjacent inoculation that may occur as a result of endospore release from traumatized polyps.1,4,9-11 Promising developments are being made in laser and endoscopic excision, as these may be the future mainstays of rhinosporidiosis therapy.1
Key Points
• Rhinosporidiosis is a chronic, granulomatous infection of the mucous membranes caused by an etiologic agent with a long-debated taxonomic classification.
• The typical clinical presentation consists of a tiny papule, most commonly of the nasal mucosa that slowly develops into a vascular, friable, violaceous, polyploid mass studded with tiny white sporangia.
• Histological examination reveals thick-walled sporangia, with many containing smaller endospores, surrounded by inflammatory infiltrate.
• Although visualizing imprint or aspiration cytology smears may allow for identification, histopathological evaluation with H&E, PAS, Gomori methenamine-silver or mucicarmine stains remains the diagnostic modality of choice.
• Surgical excision with electrodesiccation is the treatment of choice, but adjuvant use of dapsone helps to prevent dissemination or recurrence. n
Ms. Snyder, MS, is with the Center for Dermatology Research, and the departments of Dermatology at Wake Forest University School of Medicine in Winston-Salem, NC.
Mr. Al-Dabagh is a 4th year medical student at Case Western Reserve University School of Medicine in Cleveland, OH.
Dr. Feldman is with the Center for Dermatology Research and the Departments of Dermatology, Pathology and Public Health Sciences at Wake Forest University School of Medicine in Winston-Salem, NC.
Disclosures: The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories, L.P. Dr. Feldman is a consultant and speaker for Galderma, Stiefel/GlaxoSmithKline, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec and Bristol-Myers Squibb. Dr. Feldman has received grants from Galderma, Astellas, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec, Coria/Valeant, Pharmaderm, Ortho Pharmaceuticals, Aventis Pharmaceuticals, Roche Dermatology, 3M, Bristol-Myers Squibb, Stiefel/GlaxoSmithKline, Novartis, Medicis, Leo, HanAll Pharmaceuticals, Celgene, Basilea and Anacor and has received stock options from Photomedex. He is owner of www.DrScore.com and a founder of Causa Research.
Ms. Snyder and Mr. Al-Dabagh have no conflicts to disclose.
References
1. Das S, Kashyap B, Barua M, et al. Nasal rhinosporidiosis in humans: new interpretations and a review of the literature of this enigmatic disease. Med Mycol. 2011;49(3):311-315.
2. Kaushal S, Mathur SR, Mallick SR, Ramam M. Disseminated cutaneous, laryngeal, nasopharyngeal, and recurrent obstructive nasal rhinosporidiosis in an immunocompetent adult: a case report and review of literature. Int J Dermatol. 2011;50(3):340-342.
3. Kumari R, Nath AK, Rajalakshmi R, Adityan B, Thappa DM. Disseminated cutaneous rhinosporidiosis: varied morphological appearances on the skin. Indian J Dermatol Venereol Leprol. 2009;75(1):68-71.
4. Sudarshan V, Gahine R, Daharwal A, et al. Rhinosporidiosis of the parotid duct presenting as a parotid duct cyst - a report of three cases. Indian J Med Microbiol. 2012;30(1):108-111.
5. Madke B, Mahajan S, Kharkar V, Chikhalkar S, Khopkar U. Disseminated cutaneous rhinosporidiosis: varied morphological appearances on the skin. Australas J Dermatol. 2011;52(2):e4-e6.
6. Ghorpade AK. Gigantic cutaneous rhinosporidiosis with giant cells bloated with sporangia. Indian J Dermatol Venereol Leprol. 2011;77(4):517-519.
7. Verma R, Vasudevan B, Pragasam V, Deb P, Langer V, Rajagopalan S. A case of disseminated cutaneous rhinosporidiosis presenting with multiple subcutaneous nodules and a warty growth. Indian J Dermatol Venereol Leprol. 2012;78(4):520.
8. Vilela R, Mendoza L. The taxonomy and phylogenetics of the human and animal pathogen Rhinosporidium seeberei: a critical review. Rev Iberoam Micol. 2012;29(4):185-199.
9. Nayak S, Acharjya B, Devi B, Sahoo A, Singh N. Disseminated cutaneous rhinosporidiosis. Indian J Dermatol Venereol Leprol. 2007;73(3):185-187.
10. Tolat SN, Gokhale NR, Belgaumkar VA, Pradhan SN, Birud NR. Disseminated cutaneous rhinosporidiomas in an immunocompetent male. Indian J Dermatol Venereol Leprol. 2007;73(5):343-345.
11. Shenoy MM, Girisha BS, Bhandari SK, Peter R. Cutaneous rhinosporidiosis. Indian J Dermatol Venereol Leprol. 2007;73(3):179-181.
Dr. Graham was the former head of dermatopathology at the Armed Forces Institute of Pathology and the former chair of dermatology at UC Irvine in Irvine, CA. He has made available his personal library of kodachromes, slides and lectures collected throughout his career with a generous donation to the Wake Forest University Baptist Medical Center Library. Dr. Graham’s lectures and personal images in dermatomycology will be highlighted in this series to refresh the practicing clinician on deep cutaneous mycotic disease.
Rhinosporidiosis is a chronic, granulomatous mucous membrane infection presenting most commonly as a polyploid mass of the nasal, oral, ocular or genital mucosa.1 The taxonomy of the etiologic agent responsible for the disease has been long debated. First described in 1896 as a sporozoan by Guillermo Seeber, it was believed for several years to be a fungus and given the name Rhinosporidium seeberi by J.H. Ashworth in 1923.2,3 Recently, sequence analysis of an 18S small ribosomal DNA subunit has again redirected the debate. Due to this finding, the infectious agent is now grouped among a clade of fish parasites known as Mesomycetozoea.4 While its taxonomic position still remains unclear, this latest theory appears to be the most favored by researchers. Cutaneous involvement is rare and usually occurs after dissemination, arising from autoinoculation, direct implantation or hematogenous spread.2,5 Infection can cause significant morbidity in the form of functional obstruction, cosmetic injury and recurrence. Although the occurrence of systemic disease is rare, it may result in wasting, fever or even death secondary to lesions of the liver, kidneys, lungs, bone or spleen.1 To reduce morbidity and relieve any chance of mortality due to this disease, prompt recognition and adequate treatment are necessary.2
Epidemiology and Pathogenesis
R. seeberi, long believed to be of fungal origin, is now theorized to be an aquatic protistan parasite of the Mesomycetozoea class. It is thought to be contracted via contaminated water, dust or fomites.6 Although reported in Europe, Africa and the Americas, rhinosporidiosis is endemic in India and Sri Lanka.1,4,6 Migration from Asian countries to the West has led to a prevalence in the tropics.1 Bathing in stagnant or contaminated fresh water, a common practice in the Indian subcontinent, appears to be a risk factor.5-7 Although women tend to present more frequently with ocular involvement, a higher incidence of the most common presentation, nasal and nasopharyngeal infection, is seen in males age 20 to 40 years.3,6
A traumatized epithelium allows for a direct inoculation of the infectious agent, which produces endosporulating cells in the infected host and forms thick-walled sporangia within host subepithelium.1 Mature forms also contain smaller endospores (Figure 1).1,8 Following infection, dissemination may occur via autoinoculation or hematogenous spread. The former, usually the product of scratching, leads to satellite lesions adjacent to the primary site, while the latter may initiate spread to anatomically distant sites or fulminant systemic involvement.9

Figure 1. Rhinosporidiosis sporangia with endospores. Source: Graham Library of Digital Images, Wake Forest University Department
of Dermatology © 2009 Wake Forest University Dermatology
Clinical Presentation
Cutaneous lesions begin as tiny papules, and slowly evolve into large warty, poly-poidal masses.10 Nasal involvement can lead to nasal obstruction and bleeding, as masses can become quite large and tend to be very friable and vascular. The pedunculated or sessile polyp will have a purple-red surface studded with small white dots representing the sporangium within the epithelium. This pattern gives the lesion its characteristic strawberry-like appearance.1,3
Histopathology
A biopsy reveals chronic lymphocytic infiltrate along with the presence of plasma cells and foreign body giant cells (Figure 2).2

Figure 2. Rhinosporidiosis biopsy showing inflammatory infiltrate and characteristic sporangia. Source: Graham Library of Digital Images, Wake Forest University Department of Dermatology © 2009 Wake Forest University Dermatology
Within the subepithelial connective tissue of the host, a large number of thick, chitinous-walled, globular sporangia may be seen in various sizes and stages of maturation (10->450 µm). Many of these sporangia contain numerous endospores (6-12 µm), but those lacking maturity or having collapsed do not (Figure 3).2,3

Figure 3. Mix of mature (containing endospores), immature and collapsed sporangia within host subepithelium. Source: Graham
Library of Digital Images, Wake Forest University Department of Dermatology © 2009 Wake Forest University Dermatology
Differential Diagnosis
Because cutaneous rhinosporidiosis is so rare, a high degree of clinical suspicion and histopathological evaluation is required to differentiate the illness from other mimickers. It may be confused with a long list of diseases presenting with similar-appearing papules or masses including verruca vulgaris, coccidioidomycosis, bacillary angiomatosis, pyogenic granuloma, verrucous tuberculosis, donovanosis and ecthyma (Table).3,6,7,9 Of these, rhinosporidiosis is most commonly mistaken for coccidioidomycosis. However, morphology, patient history and histological presentation draw a clear distinction between the two. While the pair share a histological similarity in that they both possess sporangia, the spores of coccidioidomycosis are much smaller (<60 µm in diameter).3
Diagnostic Tests
R seeberi growth in culture is ineffective, and therefore, other means of identification are necessary, including fine-needle aspiration, imprint cytology and skin biopsy.5 Following aspiration cytology, examining the smear with Papanicolaou staining or 10% potassium hydroxide may allow for identification of the organism in different stages of maturation. Giemsa stained imprint smears may also prove to be an effective modality.2,9 However, histopathological examination remains the diagnostic method of choice.2,5,9 Detection of the characteristic bilamellar, thick-walled sporangia containing endospores may be accomplished using haematoxylin and eosin (H&E) stained sections. For better visualization and enhanced histopathological assessment, special stains such as Gomori methenamine-silver stain, periodic acid-Schiff (PAS) stain and mucicarmine stain should be used.4
Treatment
In general, the prognosis of cutaneous rhinosporidiosis is unfavorable due to its unrelenting behavior and tendency to recur.2 Spontaneous regression rarely occurs, and extension or dissemination of lesions is a possibility, encouraging prompt treatment. The gold standard of rhinosporidiosis therapy is surgical excision with electrodesiccation.1,9,11 However, surgery brings with it the risk of hematological dissemination, hemorrhage and nasal septal perforation, and even with complete excision, lesions still have a propensity to recur.1,9 Adjuvant medical therapies, including trimethoprim/sulfadiazine, sodium stibogluconate and antifungals (such as griseofulvin and amphotericin B), have been attempted with limited success.1 Currently, dapsone is the only effective drug used as a surgical adjunct. As evidenced by light and electron microscopic studies, dapsone acts via arrest of sporangial maturation and through the promotion of stromal fibrosis. It also prevents any post-surgery colonization or adjacent inoculation that may occur as a result of endospore release from traumatized polyps.1,4,9-11 Promising developments are being made in laser and endoscopic excision, as these may be the future mainstays of rhinosporidiosis therapy.1
Key Points
• Rhinosporidiosis is a chronic, granulomatous infection of the mucous membranes caused by an etiologic agent with a long-debated taxonomic classification.
• The typical clinical presentation consists of a tiny papule, most commonly of the nasal mucosa that slowly develops into a vascular, friable, violaceous, polyploid mass studded with tiny white sporangia.
• Histological examination reveals thick-walled sporangia, with many containing smaller endospores, surrounded by inflammatory infiltrate.
• Although visualizing imprint or aspiration cytology smears may allow for identification, histopathological evaluation with H&E, PAS, Gomori methenamine-silver or mucicarmine stains remains the diagnostic modality of choice.
• Surgical excision with electrodesiccation is the treatment of choice, but adjuvant use of dapsone helps to prevent dissemination or recurrence. n
Ms. Snyder, MS, is with the Center for Dermatology Research, and the departments of Dermatology at Wake Forest University School of Medicine in Winston-Salem, NC.
Mr. Al-Dabagh is a 4th year medical student at Case Western Reserve University School of Medicine in Cleveland, OH.
Dr. Feldman is with the Center for Dermatology Research and the Departments of Dermatology, Pathology and Public Health Sciences at Wake Forest University School of Medicine in Winston-Salem, NC.
Disclosures: The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories, L.P. Dr. Feldman is a consultant and speaker for Galderma, Stiefel/GlaxoSmithKline, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec and Bristol-Myers Squibb. Dr. Feldman has received grants from Galderma, Astellas, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec, Coria/Valeant, Pharmaderm, Ortho Pharmaceuticals, Aventis Pharmaceuticals, Roche Dermatology, 3M, Bristol-Myers Squibb, Stiefel/GlaxoSmithKline, Novartis, Medicis, Leo, HanAll Pharmaceuticals, Celgene, Basilea and Anacor and has received stock options from Photomedex. He is owner of www.DrScore.com and a founder of Causa Research.
Ms. Snyder and Mr. Al-Dabagh have no conflicts to disclose.
References
1. Das S, Kashyap B, Barua M, et al. Nasal rhinosporidiosis in humans: new interpretations and a review of the literature of this enigmatic disease. Med Mycol. 2011;49(3):311-315.
2. Kaushal S, Mathur SR, Mallick SR, Ramam M. Disseminated cutaneous, laryngeal, nasopharyngeal, and recurrent obstructive nasal rhinosporidiosis in an immunocompetent adult: a case report and review of literature. Int J Dermatol. 2011;50(3):340-342.
3. Kumari R, Nath AK, Rajalakshmi R, Adityan B, Thappa DM. Disseminated cutaneous rhinosporidiosis: varied morphological appearances on the skin. Indian J Dermatol Venereol Leprol. 2009;75(1):68-71.
4. Sudarshan V, Gahine R, Daharwal A, et al. Rhinosporidiosis of the parotid duct presenting as a parotid duct cyst - a report of three cases. Indian J Med Microbiol. 2012;30(1):108-111.
5. Madke B, Mahajan S, Kharkar V, Chikhalkar S, Khopkar U. Disseminated cutaneous rhinosporidiosis: varied morphological appearances on the skin. Australas J Dermatol. 2011;52(2):e4-e6.
6. Ghorpade AK. Gigantic cutaneous rhinosporidiosis with giant cells bloated with sporangia. Indian J Dermatol Venereol Leprol. 2011;77(4):517-519.
7. Verma R, Vasudevan B, Pragasam V, Deb P, Langer V, Rajagopalan S. A case of disseminated cutaneous rhinosporidiosis presenting with multiple subcutaneous nodules and a warty growth. Indian J Dermatol Venereol Leprol. 2012;78(4):520.
8. Vilela R, Mendoza L. The taxonomy and phylogenetics of the human and animal pathogen Rhinosporidium seeberei: a critical review. Rev Iberoam Micol. 2012;29(4):185-199.
9. Nayak S, Acharjya B, Devi B, Sahoo A, Singh N. Disseminated cutaneous rhinosporidiosis. Indian J Dermatol Venereol Leprol. 2007;73(3):185-187.
10. Tolat SN, Gokhale NR, Belgaumkar VA, Pradhan SN, Birud NR. Disseminated cutaneous rhinosporidiomas in an immunocompetent male. Indian J Dermatol Venereol Leprol. 2007;73(5):343-345.
11. Shenoy MM, Girisha BS, Bhandari SK, Peter R. Cutaneous rhinosporidiosis. Indian J Dermatol Venereol Leprol. 2007;73(3):179-181.
Dr. Graham was the former head of dermatopathology at the Armed Forces Institute of Pathology and the former chair of dermatology at UC Irvine in Irvine, CA. He has made available his personal library of kodachromes, slides and lectures collected throughout his career with a generous donation to the Wake Forest University Baptist Medical Center Library. Dr. Graham’s lectures and personal images in dermatomycology will be highlighted in this series to refresh the practicing clinician on deep cutaneous mycotic disease.
Rhinosporidiosis is a chronic, granulomatous mucous membrane infection presenting most commonly as a polyploid mass of the nasal, oral, ocular or genital mucosa.1 The taxonomy of the etiologic agent responsible for the disease has been long debated. First described in 1896 as a sporozoan by Guillermo Seeber, it was believed for several years to be a fungus and given the name Rhinosporidium seeberi by J.H. Ashworth in 1923.2,3 Recently, sequence analysis of an 18S small ribosomal DNA subunit has again redirected the debate. Due to this finding, the infectious agent is now grouped among a clade of fish parasites known as Mesomycetozoea.4 While its taxonomic position still remains unclear, this latest theory appears to be the most favored by researchers. Cutaneous involvement is rare and usually occurs after dissemination, arising from autoinoculation, direct implantation or hematogenous spread.2,5 Infection can cause significant morbidity in the form of functional obstruction, cosmetic injury and recurrence. Although the occurrence of systemic disease is rare, it may result in wasting, fever or even death secondary to lesions of the liver, kidneys, lungs, bone or spleen.1 To reduce morbidity and relieve any chance of mortality due to this disease, prompt recognition and adequate treatment are necessary.2
Epidemiology and Pathogenesis
R. seeberi, long believed to be of fungal origin, is now theorized to be an aquatic protistan parasite of the Mesomycetozoea class. It is thought to be contracted via contaminated water, dust or fomites.6 Although reported in Europe, Africa and the Americas, rhinosporidiosis is endemic in India and Sri Lanka.1,4,6 Migration from Asian countries to the West has led to a prevalence in the tropics.1 Bathing in stagnant or contaminated fresh water, a common practice in the Indian subcontinent, appears to be a risk factor.5-7 Although women tend to present more frequently with ocular involvement, a higher incidence of the most common presentation, nasal and nasopharyngeal infection, is seen in males age 20 to 40 years.3,6
A traumatized epithelium allows for a direct inoculation of the infectious agent, which produces endosporulating cells in the infected host and forms thick-walled sporangia within host subepithelium.1 Mature forms also contain smaller endospores (Figure 1).1,8 Following infection, dissemination may occur via autoinoculation or hematogenous spread. The former, usually the product of scratching, leads to satellite lesions adjacent to the primary site, while the latter may initiate spread to anatomically distant sites or fulminant systemic involvement.9

Figure 1. Rhinosporidiosis sporangia with endospores. Source: Graham Library of Digital Images, Wake Forest University Department
of Dermatology © 2009 Wake Forest University Dermatology
Clinical Presentation
Cutaneous lesions begin as tiny papules, and slowly evolve into large warty, poly-poidal masses.10 Nasal involvement can lead to nasal obstruction and bleeding, as masses can become quite large and tend to be very friable and vascular. The pedunculated or sessile polyp will have a purple-red surface studded with small white dots representing the sporangium within the epithelium. This pattern gives the lesion its characteristic strawberry-like appearance.1,3
Histopathology
A biopsy reveals chronic lymphocytic infiltrate along with the presence of plasma cells and foreign body giant cells (Figure 2).2

Figure 2. Rhinosporidiosis biopsy showing inflammatory infiltrate and characteristic sporangia. Source: Graham Library of Digital Images, Wake Forest University Department of Dermatology © 2009 Wake Forest University Dermatology
Within the subepithelial connective tissue of the host, a large number of thick, chitinous-walled, globular sporangia may be seen in various sizes and stages of maturation (10->450 µm). Many of these sporangia contain numerous endospores (6-12 µm), but those lacking maturity or having collapsed do not (Figure 3).2,3

Figure 3. Mix of mature (containing endospores), immature and collapsed sporangia within host subepithelium. Source: Graham
Library of Digital Images, Wake Forest University Department of Dermatology © 2009 Wake Forest University Dermatology
Differential Diagnosis
Because cutaneous rhinosporidiosis is so rare, a high degree of clinical suspicion and histopathological evaluation is required to differentiate the illness from other mimickers. It may be confused with a long list of diseases presenting with similar-appearing papules or masses including verruca vulgaris, coccidioidomycosis, bacillary angiomatosis, pyogenic granuloma, verrucous tuberculosis, donovanosis and ecthyma (Table).3,6,7,9 Of these, rhinosporidiosis is most commonly mistaken for coccidioidomycosis. However, morphology, patient history and histological presentation draw a clear distinction between the two. While the pair share a histological similarity in that they both possess sporangia, the spores of coccidioidomycosis are much smaller (<60 µm in diameter).3
Diagnostic Tests
R seeberi growth in culture is ineffective, and therefore, other means of identification are necessary, including fine-needle aspiration, imprint cytology and skin biopsy.5 Following aspiration cytology, examining the smear with Papanicolaou staining or 10% potassium hydroxide may allow for identification of the organism in different stages of maturation. Giemsa stained imprint smears may also prove to be an effective modality.2,9 However, histopathological examination remains the diagnostic method of choice.2,5,9 Detection of the characteristic bilamellar, thick-walled sporangia containing endospores may be accomplished using haematoxylin and eosin (H&E) stained sections. For better visualization and enhanced histopathological assessment, special stains such as Gomori methenamine-silver stain, periodic acid-Schiff (PAS) stain and mucicarmine stain should be used.4
Treatment
In general, the prognosis of cutaneous rhinosporidiosis is unfavorable due to its unrelenting behavior and tendency to recur.2 Spontaneous regression rarely occurs, and extension or dissemination of lesions is a possibility, encouraging prompt treatment. The gold standard of rhinosporidiosis therapy is surgical excision with electrodesiccation.1,9,11 However, surgery brings with it the risk of hematological dissemination, hemorrhage and nasal septal perforation, and even with complete excision, lesions still have a propensity to recur.1,9 Adjuvant medical therapies, including trimethoprim/sulfadiazine, sodium stibogluconate and antifungals (such as griseofulvin and amphotericin B), have been attempted with limited success.1 Currently, dapsone is the only effective drug used as a surgical adjunct. As evidenced by light and electron microscopic studies, dapsone acts via arrest of sporangial maturation and through the promotion of stromal fibrosis. It also prevents any post-surgery colonization or adjacent inoculation that may occur as a result of endospore release from traumatized polyps.1,4,9-11 Promising developments are being made in laser and endoscopic excision, as these may be the future mainstays of rhinosporidiosis therapy.1
Key Points
• Rhinosporidiosis is a chronic, granulomatous infection of the mucous membranes caused by an etiologic agent with a long-debated taxonomic classification.
• The typical clinical presentation consists of a tiny papule, most commonly of the nasal mucosa that slowly develops into a vascular, friable, violaceous, polyploid mass studded with tiny white sporangia.
• Histological examination reveals thick-walled sporangia, with many containing smaller endospores, surrounded by inflammatory infiltrate.
• Although visualizing imprint or aspiration cytology smears may allow for identification, histopathological evaluation with H&E, PAS, Gomori methenamine-silver or mucicarmine stains remains the diagnostic modality of choice.
• Surgical excision with electrodesiccation is the treatment of choice, but adjuvant use of dapsone helps to prevent dissemination or recurrence. n
Ms. Snyder, MS, is with the Center for Dermatology Research, and the departments of Dermatology at Wake Forest University School of Medicine in Winston-Salem, NC.
Mr. Al-Dabagh is a 4th year medical student at Case Western Reserve University School of Medicine in Cleveland, OH.
Dr. Feldman is with the Center for Dermatology Research and the Departments of Dermatology, Pathology and Public Health Sciences at Wake Forest University School of Medicine in Winston-Salem, NC.
Disclosures: The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories, L.P. Dr. Feldman is a consultant and speaker for Galderma, Stiefel/GlaxoSmithKline, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec and Bristol-Myers Squibb. Dr. Feldman has received grants from Galderma, Astellas, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec, Coria/Valeant, Pharmaderm, Ortho Pharmaceuticals, Aventis Pharmaceuticals, Roche Dermatology, 3M, Bristol-Myers Squibb, Stiefel/GlaxoSmithKline, Novartis, Medicis, Leo, HanAll Pharmaceuticals, Celgene, Basilea and Anacor and has received stock options from Photomedex. He is owner of www.DrScore.com and a founder of Causa Research.
Ms. Snyder and Mr. Al-Dabagh have no conflicts to disclose.
References
1. Das S, Kashyap B, Barua M, et al. Nasal rhinosporidiosis in humans: new interpretations and a review of the literature of this enigmatic disease. Med Mycol. 2011;49(3):311-315.
2. Kaushal S, Mathur SR, Mallick SR, Ramam M. Disseminated cutaneous, laryngeal, nasopharyngeal, and recurrent obstructive nasal rhinosporidiosis in an immunocompetent adult: a case report and review of literature. Int J Dermatol. 2011;50(3):340-342.
3. Kumari R, Nath AK, Rajalakshmi R, Adityan B, Thappa DM. Disseminated cutaneous rhinosporidiosis: varied morphological appearances on the skin. Indian J Dermatol Venereol Leprol. 2009;75(1):68-71.
4. Sudarshan V, Gahine R, Daharwal A, et al. Rhinosporidiosis of the parotid duct presenting as a parotid duct cyst - a report of three cases. Indian J Med Microbiol. 2012;30(1):108-111.
5. Madke B, Mahajan S, Kharkar V, Chikhalkar S, Khopkar U. Disseminated cutaneous rhinosporidiosis: varied morphological appearances on the skin. Australas J Dermatol. 2011;52(2):e4-e6.
6. Ghorpade AK. Gigantic cutaneous rhinosporidiosis with giant cells bloated with sporangia. Indian J Dermatol Venereol Leprol. 2011;77(4):517-519.
7. Verma R, Vasudevan B, Pragasam V, Deb P, Langer V, Rajagopalan S. A case of disseminated cutaneous rhinosporidiosis presenting with multiple subcutaneous nodules and a warty growth. Indian J Dermatol Venereol Leprol. 2012;78(4):520.
8. Vilela R, Mendoza L. The taxonomy and phylogenetics of the human and animal pathogen Rhinosporidium seeberei: a critical review. Rev Iberoam Micol. 2012;29(4):185-199.
9. Nayak S, Acharjya B, Devi B, Sahoo A, Singh N. Disseminated cutaneous rhinosporidiosis. Indian J Dermatol Venereol Leprol. 2007;73(3):185-187.
10. Tolat SN, Gokhale NR, Belgaumkar VA, Pradhan SN, Birud NR. Disseminated cutaneous rhinosporidiomas in an immunocompetent male. Indian J Dermatol Venereol Leprol. 2007;73(5):343-345.
11. Shenoy MM, Girisha BS, Bhandari SK, Peter R. Cutaneous rhinosporidiosis. Indian J Dermatol Venereol Leprol. 2007;73(3):179-181.





























