Non-Surgical Treatment for Basal Cell Carcinoma
The National Cancer Institute estimates that >1.5 million new cases of basal cell carcinoma (BCC) were diagnosed in the United States in 2012. BCCs are generally slow-growing tumors without significant extension. With early intervention, most low-to-moderate risk BCCs can be treated surgically with 5-year cure rates >95%. However, BCCs in some patients are unsuitable for surgery or the patient may prefer a non-surgical treatment. In an article in Journal of Drugs in Dermatology, Goldenberg et al examined non-surgical approaches to BCC, with particular focus on the management of advanced BCCs.
Radiotherapy is a non-surgical option for the primary treatment of either low- or high-risk BCCs. A limited number of studies showed that radiotherapy is generally considered the second most effective treatment option for BCC behind surgery. Although 5-year BCC cure rates with radiotherapy are commonly reported to be >90%, lack of margin control is a concern, particularly for poorly defined tumors. Radiotherapy has also been associated with increased risk of subsequent BCC, other skin cancers and sarcomas.
For BCC patients in which surgery or radiation is contraindicated or impractical, the 2012 National Clinical Practice Network guidelines identify topical pharmacotherapies and photodynamic therapy (PDT) as superficial therapies. These therapies are generally associated with somewhat lower clearance rates and/or higher recurrence rates compared with surgery or radiotherapy. Currently, 2 topical pharmacotherapies are FDA approved for superficial BCC: imiquimod (Aldara) and 5-fluorouracil (Efudex). A positive feature of PDT is goodcosmesis, but the therapy is not FDA approved or well standardized, say researchers.
Vismodegib (Erivedge), a first-in-class hedgehog pathway inhibitor (HHI), is a recent addition to the limited armamentarium FDA approved for the treatment of adults with metastatic BCC, or those with locally advanced BCC that has recurred following surgery or in patinets who are not candidates for surgery and who are not candidates for radiation. Approval of vismodegib for the treatment of advanced BCC was primarly based on the findings from a multicenter, international, 2-cohort, non-randomized, Phase II study of vismodegib 150 mg daily in patients with metastatic BCC (N=33) or locally advanced BCC who had inoperable disease for whom surgery was inappropriate (N=63). The median duration of response was 7.6 months in both groups. Patients in the metastatic BCC group (64%) and 38% in the locally advanced BCC groups had stable disease as best response. Therefore, 94% of metastatic BCC patients and 84% of locally advanced BCC patients appeared to achieve some benefit with this therapy. The most common adverse events across both groups were muscle spasms, alopecia, dysgeusia, weight loss and fatigue.
In addition to vismodegib, a number of other HHIs are in development for use alone or in combination for BCC and other tumors. These include LDE-225, LEQ-506, BMS-833923 and PF-04449913.
Goldenberg G, Hamid O. Nonsurgical treatment options for basal cell carcinoma--focus on advanced disease. J Drugs Dermatol. 2013;12(12):1369-1378.
Dermoscopic Criteria to Predict Residual Disease
 In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
For the study, lesions exhibiting RDADC 3 months after treatment were biopsied and in case of histopathologic confirmation were excised. Lesions characterized by white/red structureless areas, superficial fine telangiectasias or lacking any dermoscopic criterion were monitored for 12 months.
At the 3-month evaluation, the results detected ≥1 RDADC in 25.5% of superficial BCCs, in which histology confirmed tumor presence. In 45 of the 73 remaining lesions, dermoscopy revealed red/white structureless areas and/or superficial fine telangiectasias; 28 lacked any dermoscopic criterion of superficial BCC. In total, disease recurred in 13 of the 73 lesions (17.8%).
This study demonstrated that RDADC accurately predict residual disease; and the absence of dermoscopic criteria of superficial BCC safely predicts complete histopathologic clearance. The researchers note that “detection of red/white structureless areas and/or superficial fine telangiectasias warrants close monitoring to recognize early recurrence.” n
Apalla Z, Lallas A, Tzellos T, et al. Applicability of dermoscopy for evaluation of patients’ response to non-ablative therapies for the treatment of superficial basal cell carcinoma. Br J Dermatol. Published online ahead of print November 27, 2013.
Vismodegib for Advanced Basal Cell Carcinoma
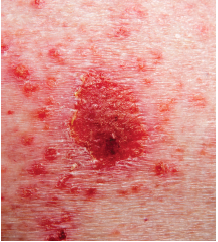 Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Because additional clinical data are critical to confirm the efficacy and safety of vismodegib, Chang et al conducted an open-label, multicenter study in patients with advanced BCC inappropriate for radiotherapy or surgery. The study included 119 patients with advanced BCC who received 150 mg vismodegib daily until disease progression or intolerable toxicity. Tumor response was assessed via Response Evaluation Criteria in Solid Tumors version 1.0. The study cohort took vismodegib for a median of 5.5 months. The mean follow-up for safety was 6.5 months.
The study results showed that objective responses occurred in 46.6% of locally advanced BCC and 30.8% of patients with metastatic BCC. Response was negatively associated with prior systemic therapy in patients with locally advanced BCC (P=.002). The most common adverse events reported were muscle spasms (70.6%), dysgeusia (70.6%), alopecia (58.0%) and diarrhea (25.2%).
The investigators note that the abbreviated follow-up time because of study termination upon FDA approval was a study limitation.
Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70(1):60-69.
Questionnaires Measure Patient-Reported Outcomes
 Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
To develop the questionnaires, the researchers conducted concept elicitation interviews with patients with advancd BCC (N=14) and BCCNS (N=16) from 5 US clinical sites and the BCCNS Life Support Network and 4 physicians. The patient-reported outcome questionnaires were drafted based on the results from a literature review and the findings from these interviews.
Reporting on the results published online in JAMA Dermatology, the researchers found that most concept elicitation interview patients were male (63%) and white (93%) with a mean age of 57 years. Both patients groups said their conditions impacted emotional, social and physical functioning. Furthermore, 79% of patients with advanced BCC and all patients with BCCNS reported scarring. Physician interviews revealed similar results. The questionnaires were found to be relevant, clear and comprehensive, according to cognitive debriefing interviews.
The researchers conclude that advanced BCC and BCCNS affects patients in unique and substantial ways. These questionnaires were developed with patient and clinician input and measure the critical areas that have a negative effects on patients with these conditions. n
Mathias SD, Chren MM, Colwell HH, et al. Assessing health-related quality of life for advanced basal cell carcinoma and basal cell carcinoma nevus syndrome: development of the first disease-specific patient-reported outcome questionnaires. JAMA Dermatol. Published online ahead of print November 27, 2013.
Photodynamic Diagnosis Inceases Surgical Efficacy
B ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
For the study, the researchers examined 67 cases of biopsy-proven primary facial cutaneous SCC treated with MMS. The cases were divided into 2 groups depending on PDD application. The PDD group included 38 cases and the non-PDD group included 29 cases. The primary endpoint was the differences in surgical features between both groups.
The results showed that the PDD technique can increase surgical efficacy of primary cutaneous SCC during MMS. The PDD group needed fewer Mohs stages compared with the non-PDD group (1.37 vs 1.83, respectively; P=.02) and smaller sugical incisions (8.03 vs 11.24 mm, respectively; P=.03) The benefits of PDD were more pronounced in low-risk SCC in terms of surgical margins and Mohs stage, including thin (≤4 mm), small (≤20 mm), well-differentiated and non-ulcerative tumors (P<.05).
The researchers conclude that PDD is simple and useful technique for delineating the margins of low-risk SCC before MMS.
Jeon SY, Kim KH, Song KH. Efficacy of photodynamic diagnosis-guided Mohs micrographic surgery in primary squamous cell carcinoma. Dermatol Surg. 2013;39(12):1774-1783.
Combination Demonstrates Improvement in Squamous Cell Carcinoma
 Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
The researchers evaluated the anti-proliferative and anti-tumor activities of the cetuximab-namitecan combination in the SCC models characterized by a different EGFR gene copy number/EGFR protein level. The authors also assessed the effects of the combination on EGFR expression at both mRNA and protein levels and investigated the molecular basis of the interaction between the 2 agents.
The findings, published in Clinical Cancer Research, showed that cetuximab and namitecan exhibited synergistic effects, resulting in potentiation of cell growth inhibition. The combination of both agents enhanced therapeutic efficacy, with high cure rates in 3 SCC models characterized by high EGFR gene copy number, without increasing toxicity. The researchers also observed the synergistic anti-tumor effect with the cetuximab-irinotecan combination. At the molecular level, the 2 agents produced a cooperative effect leading to complete down-regulation of EGFR. An interesting finding noted by the researchers, was that when singly administered, the camptothecin was able to strongly decrease EGFR expression mainly by transcriptional inhibition.
“Our results demonstrate a marked efficacy of the cetuximab/namitecan combination, which reflects a complete abrogation of EGFR expression as a critical determinant of the therapeutic improvement, in SCC preclinical models, and suggest EGFR gene copy number as a possible marker to be used for patient selection in the clinical setting,” conclude the researchers.
Zuco V, De Cesare M, Lauricella C, et al. Syngergistic antitumor activity of cetuximab and namitecan in human squamous cell carcinoma models relies on coppoertive inhibition of EGFR expression and depends on high EGFR gene copy number. Clin Cancer Res. Published online ahead of print December 10, 2013.
BRAF Mutation Among Young Adults with Melanoma
 Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma was more significant in female patients (61.4%), the trunk was the most common site of involvement (40.4%) and superficially spreading melanoma was the predominant histologic type (79.5%), according to results published in Diagnostic Molecular Pathology. The researchers successfully analyzed mutation in BRAF gene in 93 cases using an RT-PCR. The BRAF gene mutation was identified in 36 cases (38.7%) and was associated with vertical growth phase (P=0.01) and mild inflammatory infiltrate (P=0.02). No case of melanoma with regression phenomenon presented with BRAF gene mutation (P<0.05). The findings also showed no significant association between BRAF gene mutation and sex, histologic type, the Clark level, the Breslow index, solar elastosis, angiolymphatic and perineural invasion, satellitosis and coexisting nevus.
As in melanoma in elderly patients, the researchers conclude that these results most likely indicate that BRAF gene mutation may not be the only critical factor in melanoma tumorigenesis, and that there should be multiple alternative genetic pathways related to melanoma. n
Estrozi B, Michado J, Rodriguez R, Bacchi CE. Clinicopathologic findings and BRAF mutation in cutaneous melanoma in young adults. Diagn Mol Pathol. 2014;22(1):57-64.
To read more news, trends and research updates about skin cancer, visit www.the-dermatologist.com/skin-cancer.
Non-Surgical Treatment for Basal Cell Carcinoma
The National Cancer Institute estimates that >1.5 million new cases of basal cell carcinoma (BCC) were diagnosed in the United States in 2012. BCCs are generally slow-growing tumors without significant extension. With early intervention, most low-to-moderate risk BCCs can be treated surgically with 5-year cure rates >95%. However, BCCs in some patients are unsuitable for surgery or the patient may prefer a non-surgical treatment. In an article in Journal of Drugs in Dermatology, Goldenberg et al examined non-surgical approaches to BCC, with particular focus on the management of advanced BCCs.
Radiotherapy is a non-surgical option for the primary treatment of either low- or high-risk BCCs. A limited number of studies showed that radiotherapy is generally considered the second most effective treatment option for BCC behind surgery. Although 5-year BCC cure rates with radiotherapy are commonly reported to be >90%, lack of margin control is a concern, particularly for poorly defined tumors. Radiotherapy has also been associated with increased risk of subsequent BCC, other skin cancers and sarcomas.
For BCC patients in which surgery or radiation is contraindicated or impractical, the 2012 National Clinical Practice Network guidelines identify topical pharmacotherapies and photodynamic therapy (PDT) as superficial therapies. These therapies are generally associated with somewhat lower clearance rates and/or higher recurrence rates compared with surgery or radiotherapy. Currently, 2 topical pharmacotherapies are FDA approved for superficial BCC: imiquimod (Aldara) and 5-fluorouracil (Efudex). A positive feature of PDT is goodcosmesis, but the therapy is not FDA approved or well standardized, say researchers.
Vismodegib (Erivedge), a first-in-class hedgehog pathway inhibitor (HHI), is a recent addition to the limited armamentarium FDA approved for the treatment of adults with metastatic BCC, or those with locally advanced BCC that has recurred following surgery or in patinets who are not candidates for surgery and who are not candidates for radiation. Approval of vismodegib for the treatment of advanced BCC was primarly based on the findings from a multicenter, international, 2-cohort, non-randomized, Phase II study of vismodegib 150 mg daily in patients with metastatic BCC (N=33) or locally advanced BCC who had inoperable disease for whom surgery was inappropriate (N=63). The median duration of response was 7.6 months in both groups. Patients in the metastatic BCC group (64%) and 38% in the locally advanced BCC groups had stable disease as best response. Therefore, 94% of metastatic BCC patients and 84% of locally advanced BCC patients appeared to achieve some benefit with this therapy. The most common adverse events across both groups were muscle spasms, alopecia, dysgeusia, weight loss and fatigue.
In addition to vismodegib, a number of other HHIs are in development for use alone or in combination for BCC and other tumors. These include LDE-225, LEQ-506, BMS-833923 and PF-04449913.
Goldenberg G, Hamid O. Nonsurgical treatment options for basal cell carcinoma--focus on advanced disease. J Drugs Dermatol. 2013;12(12):1369-1378.
Dermoscopic Criteria to Predict Residual Disease
 In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
For the study, lesions exhibiting RDADC 3 months after treatment were biopsied and in case of histopathologic confirmation were excised. Lesions characterized by white/red structureless areas, superficial fine telangiectasias or lacking any dermoscopic criterion were monitored for 12 months.
At the 3-month evaluation, the results detected ≥1 RDADC in 25.5% of superficial BCCs, in which histology confirmed tumor presence. In 45 of the 73 remaining lesions, dermoscopy revealed red/white structureless areas and/or superficial fine telangiectasias; 28 lacked any dermoscopic criterion of superficial BCC. In total, disease recurred in 13 of the 73 lesions (17.8%).
This study demonstrated that RDADC accurately predict residual disease; and the absence of dermoscopic criteria of superficial BCC safely predicts complete histopathologic clearance. The researchers note that “detection of red/white structureless areas and/or superficial fine telangiectasias warrants close monitoring to recognize early recurrence.” n
Apalla Z, Lallas A, Tzellos T, et al. Applicability of dermoscopy for evaluation of patients’ response to non-ablative therapies for the treatment of superficial basal cell carcinoma. Br J Dermatol. Published online ahead of print November 27, 2013.
Vismodegib for Advanced Basal Cell Carcinoma
 Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Because additional clinical data are critical to confirm the efficacy and safety of vismodegib, Chang et al conducted an open-label, multicenter study in patients with advanced BCC inappropriate for radiotherapy or surgery. The study included 119 patients with advanced BCC who received 150 mg vismodegib daily until disease progression or intolerable toxicity. Tumor response was assessed via Response Evaluation Criteria in Solid Tumors version 1.0. The study cohort took vismodegib for a median of 5.5 months. The mean follow-up for safety was 6.5 months.
The study results showed that objective responses occurred in 46.6% of locally advanced BCC and 30.8% of patients with metastatic BCC. Response was negatively associated with prior systemic therapy in patients with locally advanced BCC (P=.002). The most common adverse events reported were muscle spasms (70.6%), dysgeusia (70.6%), alopecia (58.0%) and diarrhea (25.2%).
The investigators note that the abbreviated follow-up time because of study termination upon FDA approval was a study limitation.
Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70(1):60-69.
Questionnaires Measure Patient-Reported Outcomes
 Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
To develop the questionnaires, the researchers conducted concept elicitation interviews with patients with advancd BCC (N=14) and BCCNS (N=16) from 5 US clinical sites and the BCCNS Life Support Network and 4 physicians. The patient-reported outcome questionnaires were drafted based on the results from a literature review and the findings from these interviews.
Reporting on the results published online in JAMA Dermatology, the researchers found that most concept elicitation interview patients were male (63%) and white (93%) with a mean age of 57 years. Both patients groups said their conditions impacted emotional, social and physical functioning. Furthermore, 79% of patients with advanced BCC and all patients with BCCNS reported scarring. Physician interviews revealed similar results. The questionnaires were found to be relevant, clear and comprehensive, according to cognitive debriefing interviews.
The researchers conclude that advanced BCC and BCCNS affects patients in unique and substantial ways. These questionnaires were developed with patient and clinician input and measure the critical areas that have a negative effects on patients with these conditions. n
Mathias SD, Chren MM, Colwell HH, et al. Assessing health-related quality of life for advanced basal cell carcinoma and basal cell carcinoma nevus syndrome: development of the first disease-specific patient-reported outcome questionnaires. JAMA Dermatol. Published online ahead of print November 27, 2013.
Photodynamic Diagnosis Inceases Surgical Efficacy
B ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
For the study, the researchers examined 67 cases of biopsy-proven primary facial cutaneous SCC treated with MMS. The cases were divided into 2 groups depending on PDD application. The PDD group included 38 cases and the non-PDD group included 29 cases. The primary endpoint was the differences in surgical features between both groups.
The results showed that the PDD technique can increase surgical efficacy of primary cutaneous SCC during MMS. The PDD group needed fewer Mohs stages compared with the non-PDD group (1.37 vs 1.83, respectively; P=.02) and smaller sugical incisions (8.03 vs 11.24 mm, respectively; P=.03) The benefits of PDD were more pronounced in low-risk SCC in terms of surgical margins and Mohs stage, including thin (≤4 mm), small (≤20 mm), well-differentiated and non-ulcerative tumors (P<.05).
The researchers conclude that PDD is simple and useful technique for delineating the margins of low-risk SCC before MMS.
Jeon SY, Kim KH, Song KH. Efficacy of photodynamic diagnosis-guided Mohs micrographic surgery in primary squamous cell carcinoma. Dermatol Surg. 2013;39(12):1774-1783.
Combination Demonstrates Improvement in Squamous Cell Carcinoma
 Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
The researchers evaluated the anti-proliferative and anti-tumor activities of the cetuximab-namitecan combination in the SCC models characterized by a different EGFR gene copy number/EGFR protein level. The authors also assessed the effects of the combination on EGFR expression at both mRNA and protein levels and investigated the molecular basis of the interaction between the 2 agents.
The findings, published in Clinical Cancer Research, showed that cetuximab and namitecan exhibited synergistic effects, resulting in potentiation of cell growth inhibition. The combination of both agents enhanced therapeutic efficacy, with high cure rates in 3 SCC models characterized by high EGFR gene copy number, without increasing toxicity. The researchers also observed the synergistic anti-tumor effect with the cetuximab-irinotecan combination. At the molecular level, the 2 agents produced a cooperative effect leading to complete down-regulation of EGFR. An interesting finding noted by the researchers, was that when singly administered, the camptothecin was able to strongly decrease EGFR expression mainly by transcriptional inhibition.
“Our results demonstrate a marked efficacy of the cetuximab/namitecan combination, which reflects a complete abrogation of EGFR expression as a critical determinant of the therapeutic improvement, in SCC preclinical models, and suggest EGFR gene copy number as a possible marker to be used for patient selection in the clinical setting,” conclude the researchers.
Zuco V, De Cesare M, Lauricella C, et al. Syngergistic antitumor activity of cetuximab and namitecan in human squamous cell carcinoma models relies on coppoertive inhibition of EGFR expression and depends on high EGFR gene copy number. Clin Cancer Res. Published online ahead of print December 10, 2013.
BRAF Mutation Among Young Adults with Melanoma
 Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma was more significant in female patients (61.4%), the trunk was the most common site of involvement (40.4%) and superficially spreading melanoma was the predominant histologic type (79.5%), according to results published in Diagnostic Molecular Pathology. The researchers successfully analyzed mutation in BRAF gene in 93 cases using an RT-PCR. The BRAF gene mutation was identified in 36 cases (38.7%) and was associated with vertical growth phase (P=0.01) and mild inflammatory infiltrate (P=0.02). No case of melanoma with regression phenomenon presented with BRAF gene mutation (P<0.05). The findings also showed no significant association between BRAF gene mutation and sex, histologic type, the Clark level, the Breslow index, solar elastosis, angiolymphatic and perineural invasion, satellitosis and coexisting nevus.
As in melanoma in elderly patients, the researchers conclude that these results most likely indicate that BRAF gene mutation may not be the only critical factor in melanoma tumorigenesis, and that there should be multiple alternative genetic pathways related to melanoma. n
Estrozi B, Michado J, Rodriguez R, Bacchi CE. Clinicopathologic findings and BRAF mutation in cutaneous melanoma in young adults. Diagn Mol Pathol. 2014;22(1):57-64.
To read more news, trends and research updates about skin cancer, visit www.the-dermatologist.com/skin-cancer.
Non-Surgical Treatment for Basal Cell Carcinoma
The National Cancer Institute estimates that >1.5 million new cases of basal cell carcinoma (BCC) were diagnosed in the United States in 2012. BCCs are generally slow-growing tumors without significant extension. With early intervention, most low-to-moderate risk BCCs can be treated surgically with 5-year cure rates >95%. However, BCCs in some patients are unsuitable for surgery or the patient may prefer a non-surgical treatment. In an article in Journal of Drugs in Dermatology, Goldenberg et al examined non-surgical approaches to BCC, with particular focus on the management of advanced BCCs.
Radiotherapy is a non-surgical option for the primary treatment of either low- or high-risk BCCs. A limited number of studies showed that radiotherapy is generally considered the second most effective treatment option for BCC behind surgery. Although 5-year BCC cure rates with radiotherapy are commonly reported to be >90%, lack of margin control is a concern, particularly for poorly defined tumors. Radiotherapy has also been associated with increased risk of subsequent BCC, other skin cancers and sarcomas.
For BCC patients in which surgery or radiation is contraindicated or impractical, the 2012 National Clinical Practice Network guidelines identify topical pharmacotherapies and photodynamic therapy (PDT) as superficial therapies. These therapies are generally associated with somewhat lower clearance rates and/or higher recurrence rates compared with surgery or radiotherapy. Currently, 2 topical pharmacotherapies are FDA approved for superficial BCC: imiquimod (Aldara) and 5-fluorouracil (Efudex). A positive feature of PDT is goodcosmesis, but the therapy is not FDA approved or well standardized, say researchers.
Vismodegib (Erivedge), a first-in-class hedgehog pathway inhibitor (HHI), is a recent addition to the limited armamentarium FDA approved for the treatment of adults with metastatic BCC, or those with locally advanced BCC that has recurred following surgery or in patinets who are not candidates for surgery and who are not candidates for radiation. Approval of vismodegib for the treatment of advanced BCC was primarly based on the findings from a multicenter, international, 2-cohort, non-randomized, Phase II study of vismodegib 150 mg daily in patients with metastatic BCC (N=33) or locally advanced BCC who had inoperable disease for whom surgery was inappropriate (N=63). The median duration of response was 7.6 months in both groups. Patients in the metastatic BCC group (64%) and 38% in the locally advanced BCC groups had stable disease as best response. Therefore, 94% of metastatic BCC patients and 84% of locally advanced BCC patients appeared to achieve some benefit with this therapy. The most common adverse events across both groups were muscle spasms, alopecia, dysgeusia, weight loss and fatigue.
In addition to vismodegib, a number of other HHIs are in development for use alone or in combination for BCC and other tumors. These include LDE-225, LEQ-506, BMS-833923 and PF-04449913.
Goldenberg G, Hamid O. Nonsurgical treatment options for basal cell carcinoma--focus on advanced disease. J Drugs Dermatol. 2013;12(12):1369-1378.
Dermoscopic Criteria to Predict Residual Disease
 In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
For the study, lesions exhibiting RDADC 3 months after treatment were biopsied and in case of histopathologic confirmation were excised. Lesions characterized by white/red structureless areas, superficial fine telangiectasias or lacking any dermoscopic criterion were monitored for 12 months.
At the 3-month evaluation, the results detected ≥1 RDADC in 25.5% of superficial BCCs, in which histology confirmed tumor presence. In 45 of the 73 remaining lesions, dermoscopy revealed red/white structureless areas and/or superficial fine telangiectasias; 28 lacked any dermoscopic criterion of superficial BCC. In total, disease recurred in 13 of the 73 lesions (17.8%).
This study demonstrated that RDADC accurately predict residual disease; and the absence of dermoscopic criteria of superficial BCC safely predicts complete histopathologic clearance. The researchers note that “detection of red/white structureless areas and/or superficial fine telangiectasias warrants close monitoring to recognize early recurrence.” n
Apalla Z, Lallas A, Tzellos T, et al. Applicability of dermoscopy for evaluation of patients’ response to non-ablative therapies for the treatment of superficial basal cell carcinoma. Br J Dermatol. Published online ahead of print November 27, 2013.
Vismodegib for Advanced Basal Cell Carcinoma
 Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Because additional clinical data are critical to confirm the efficacy and safety of vismodegib, Chang et al conducted an open-label, multicenter study in patients with advanced BCC inappropriate for radiotherapy or surgery. The study included 119 patients with advanced BCC who received 150 mg vismodegib daily until disease progression or intolerable toxicity. Tumor response was assessed via Response Evaluation Criteria in Solid Tumors version 1.0. The study cohort took vismodegib for a median of 5.5 months. The mean follow-up for safety was 6.5 months.
The study results showed that objective responses occurred in 46.6% of locally advanced BCC and 30.8% of patients with metastatic BCC. Response was negatively associated with prior systemic therapy in patients with locally advanced BCC (P=.002). The most common adverse events reported were muscle spasms (70.6%), dysgeusia (70.6%), alopecia (58.0%) and diarrhea (25.2%).
The investigators note that the abbreviated follow-up time because of study termination upon FDA approval was a study limitation.
Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70(1):60-69.
Questionnaires Measure Patient-Reported Outcomes
 Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
To develop the questionnaires, the researchers conducted concept elicitation interviews with patients with advancd BCC (N=14) and BCCNS (N=16) from 5 US clinical sites and the BCCNS Life Support Network and 4 physicians. The patient-reported outcome questionnaires were drafted based on the results from a literature review and the findings from these interviews.
Reporting on the results published online in JAMA Dermatology, the researchers found that most concept elicitation interview patients were male (63%) and white (93%) with a mean age of 57 years. Both patients groups said their conditions impacted emotional, social and physical functioning. Furthermore, 79% of patients with advanced BCC and all patients with BCCNS reported scarring. Physician interviews revealed similar results. The questionnaires were found to be relevant, clear and comprehensive, according to cognitive debriefing interviews.
The researchers conclude that advanced BCC and BCCNS affects patients in unique and substantial ways. These questionnaires were developed with patient and clinician input and measure the critical areas that have a negative effects on patients with these conditions. n
Mathias SD, Chren MM, Colwell HH, et al. Assessing health-related quality of life for advanced basal cell carcinoma and basal cell carcinoma nevus syndrome: development of the first disease-specific patient-reported outcome questionnaires. JAMA Dermatol. Published online ahead of print November 27, 2013.
Photodynamic Diagnosis Inceases Surgical Efficacy
B ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
For the study, the researchers examined 67 cases of biopsy-proven primary facial cutaneous SCC treated with MMS. The cases were divided into 2 groups depending on PDD application. The PDD group included 38 cases and the non-PDD group included 29 cases. The primary endpoint was the differences in surgical features between both groups.
The results showed that the PDD technique can increase surgical efficacy of primary cutaneous SCC during MMS. The PDD group needed fewer Mohs stages compared with the non-PDD group (1.37 vs 1.83, respectively; P=.02) and smaller sugical incisions (8.03 vs 11.24 mm, respectively; P=.03) The benefits of PDD were more pronounced in low-risk SCC in terms of surgical margins and Mohs stage, including thin (≤4 mm), small (≤20 mm), well-differentiated and non-ulcerative tumors (P<.05).
The researchers conclude that PDD is simple and useful technique for delineating the margins of low-risk SCC before MMS.
Jeon SY, Kim KH, Song KH. Efficacy of photodynamic diagnosis-guided Mohs micrographic surgery in primary squamous cell carcinoma. Dermatol Surg. 2013;39(12):1774-1783.
Combination Demonstrates Improvement in Squamous Cell Carcinoma
 Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
The researchers evaluated the anti-proliferative and anti-tumor activities of the cetuximab-namitecan combination in the SCC models characterized by a different EGFR gene copy number/EGFR protein level. The authors also assessed the effects of the combination on EGFR expression at both mRNA and protein levels and investigated the molecular basis of the interaction between the 2 agents.
The findings, published in Clinical Cancer Research, showed that cetuximab and namitecan exhibited synergistic effects, resulting in potentiation of cell growth inhibition. The combination of both agents enhanced therapeutic efficacy, with high cure rates in 3 SCC models characterized by high EGFR gene copy number, without increasing toxicity. The researchers also observed the synergistic anti-tumor effect with the cetuximab-irinotecan combination. At the molecular level, the 2 agents produced a cooperative effect leading to complete down-regulation of EGFR. An interesting finding noted by the researchers, was that when singly administered, the camptothecin was able to strongly decrease EGFR expression mainly by transcriptional inhibition.
“Our results demonstrate a marked efficacy of the cetuximab/namitecan combination, which reflects a complete abrogation of EGFR expression as a critical determinant of the therapeutic improvement, in SCC preclinical models, and suggest EGFR gene copy number as a possible marker to be used for patient selection in the clinical setting,” conclude the researchers.
Zuco V, De Cesare M, Lauricella C, et al. Syngergistic antitumor activity of cetuximab and namitecan in human squamous cell carcinoma models relies on coppoertive inhibition of EGFR expression and depends on high EGFR gene copy number. Clin Cancer Res. Published online ahead of print December 10, 2013.
BRAF Mutation Among Young Adults with Melanoma
 Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma was more significant in female patients (61.4%), the trunk was the most common site of involvement (40.4%) and superficially spreading melanoma was the predominant histologic type (79.5%), according to results published in Diagnostic Molecular Pathology. The researchers successfully analyzed mutation in BRAF gene in 93 cases using an RT-PCR. The BRAF gene mutation was identified in 36 cases (38.7%) and was associated with vertical growth phase (P=0.01) and mild inflammatory infiltrate (P=0.02). No case of melanoma with regression phenomenon presented with BRAF gene mutation (P<0.05). The findings also showed no significant association between BRAF gene mutation and sex, histologic type, the Clark level, the Breslow index, solar elastosis, angiolymphatic and perineural invasion, satellitosis and coexisting nevus.
As in melanoma in elderly patients, the researchers conclude that these results most likely indicate that BRAF gene mutation may not be the only critical factor in melanoma tumorigenesis, and that there should be multiple alternative genetic pathways related to melanoma. n
Estrozi B, Michado J, Rodriguez R, Bacchi CE. Clinicopathologic findings and BRAF mutation in cutaneous melanoma in young adults. Diagn Mol Pathol. 2014;22(1):57-64.
To read more news, trends and research updates about skin cancer, visit www.the-dermatologist.com/skin-cancer.
 In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say. Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.  Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations. ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery. Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models. Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene. 





 In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say.
In a new study, researchers aimed to assess the hypothesis that certain dermoscopic criteria, namely pigmented structures, ulceration and arborizing vessels have been suggested to predict the presence of residual disease (residual disease-associated dermoscopic criteria [RDADC]). The findings were published online in British Journal of Dermatology. Applicability of dermoscopy in evaluation of outcome and monitoring of superficial basal cell carcinoma (BCC) following non-ablative therapies has not been sufficiently assessed, they say. Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.
Clinical data from a study published in the Journal of the American Academy of Dermatology supports the efficacy and safety of vismodegib (Erivedge) in the treatment of basal cell carcinoma (BCC). The FDA approved vismodegib in 2012 for advanced BCC based on a single, non-randomized, Phase II trial.  Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations.
Little is known about patients’ experiences of advanced basal cell carcinoma (BCC) and basal cell carcinoma nevus syndrome (BCCNS). Researchers developed 2 questionnaires to allow clinicians to assess the emotional, social and physical impacts of the disease on patients in these populations. ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery.
ecause of its irregular invasive patterns, cutaneous squamous cell carcinoma (SCC) typically has ill-defined margins. In a new study, Jeon et al evaluated the efficacy of photodynamic diagnosis (PDD) in primary cutaneous SCC treated with the Mohs micrographic surgery (MMS). The findings were published in Dermatologic Surgery. Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models.
Despite frequent overexpression of epidermal growth factor (EGFR) in squamous cell carcinoma (SCC), the efficacy of cetuximab alone is limited. Given the marked activity of namitecan against SCC models, researchers conducted a new study to assesse the efficacy of cetuximab-namitecan combination in 4 SCC models. Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene.
Cutaneous melanoma in young adults is rare with increasing incidence. It is unclear whether the etiology and clinical outcome are similar to cutaneous melanoma in elderly patients. In general, the frequency of mutations in BRAF gene in patients with cutaneous melanoma is 20%-80%; however, the status and clinical significance of BRAF mutations in the young have not been evaluated. As a result, researchers investigated 132 cases of primary cutaneous melanoma in patients age 18 to 30 focusing on clinical characteristics, pathologic features and molecular evaluation of mutation in the BRAF gene. 


















