AAD Releases Position Statement on BCC and SCC Treatment Options
The American Academy of Dermatology (AAD) recently released a position paper on superficial radiation therapy and electronic surface brachytherapy. The paper explains the technology and offers dermatologists 10 guiding principles regarding provision of superficial radiation therapy and electronic surface brachytherapy services “in order to provide high quality care for patients, but is not intended to establish a legal or medical standard of care,” according to the Academy.
The Academy notes that surgical treatment (eg, excision, Mohs, destruction) is the optimal primary intervention for basal cell carcinomas (BCC) and squamous cell carcinomas (SCC) and notes that superficial radiation therapy is a consideration as a second-line option for the treatment of BCC and SCC, for use in special circumstances, including when surgical intervention is contraindicated and after the benefits and risks of treatment alternatives have been discussed with the patient.
In addition, the Academy advises that more research is needed on superficial radiation therapy and electronic surface brachytherapy. It also notes that dermatologists providing superficial radiation therapy must have adequate education and training to administer the therapies. (Electronic surface brachytherapy should not be administered by a dermatologist, according to the current manufacturer’s recommendations.)
The statement also cautions dermatologists to be aware that “superficial radiation therapy and/or electronic surface brachytherapy devices are being marketed to dermatologists as technologically advanced devices with significant current and future revenue streams” and that the Academy’s Code of Ethics for Dermatologists “precludes patient management based on business models designed solely for the financial gain to the dermatology practice, without adequate concern for the best interests of the patient. Such an approach would undermine quality of care and compromise patient safety, and could subject the practice to ethical scrutiny.”
The Academy adds that, “It is important that any practice using superficial radiation therapy, electronic brachytherapy or similar therapies expend appropriate efforts to understand and use proper CPT coding for the service that is verified as such by an authoritative entity other than the device manufacturer.”
Please visit https://aad.org/ to read the position statement in its entirety.
Mechlorethamine gel 0.016% now available in the US
Valchlor (mechlorethamine, Actelion), the first FDA-approved topical formulation of mechlorethamine, is now available for patients in the United States. The gel, which is applied topically once a day, is an alkylating drug indicated to treat patients with stage IA and IB mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL) who have received prior skin-directed therapy. It is an orphan drug that was acquired by Actelion US Holding Company, a subsidiary of Actelion Ltd., in September 2013 as part of a merger with Ceptaris Therapeutics, Inc. The gel is distributed in the US by Accredo Specialty Pharmacy.
“The availability of Valchlor is exciting news for patients and the treatment community. Physicians now have the option to treat appropriate MF-CTCL patients with the first formulation of topical mechlorethamine that is FDA approved based on rigorous clinical evidence to support its use,” says Youn H. Kim, MD, Joanne and Peter Haas Jr. professor for cutaneous lymphoma research, professor of dermatology, and director, Multidisciplinary Cutaneous Lymphoma Clinic, Stanford University School of Medicine.
“The use of topical mechlorethamine has been documented over several decades,” explains Stuart Lessin, MD, former director of dermatology at the Fox Chase Cancer Center, president of the Board of Directors of the Cutaneous Lymphoma Foundation and lead investigator in the pivotal trial. “With the launch of Valchlor, physicians can prescribe with confidence knowing that an FDA-approved formulation is now available, along with support and financial-assistance programs for eligible patients to help facilitate education and access.”
Mycosis fungoides, the most common type of cutaneous T-cell lymphoma, is a rare form of non-Hodgkin’s lymphoma. In the Unite States, approximately 20,000 patients are diagnosed with stage IA-IB MF-CTCL, qualifying it as a rare or orphan disease.
Nominations Being Accepted for Practice Manager of the Year Award
 The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
A committee of ADAM members will review nominations to determine the inaugural recipient. The winner will receive a cash prize of $1,000, and a registration scholarship to attend the 2015 ADAM Annual Meeting. The recipient of the award will be announced at the 2014 ADAM Annual Meeting, held in Denver, Colorado, March 19-21, 2014.
“The Practice Manager of the Year recognizes those individuals who exceed all standards within the profession. We are grateful to CareCredit for its support in establishing and supporting this endeavor,” says Pam Kroussakis, executive director, the ADAM.
Article continues on page 2
{{pagebreak}}
NRS Awards New Grants for Rosacea Research
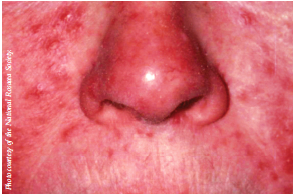 The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
Dr. Anna Di Nardo, associate professor of medicine at the University of California-San Diego, and colleagues were awarded $25,000 to study whether blocking the activity of mast cells in individuals with rosacea may alleviate inflammation. In an NRS-funded study completed earlier this year, Dr. Di Nardo’s group discovered that mast cells, located at the interface between the nervous system and the vascular system, are the “missing link” between rosacea triggers and inflammation. Positioned just below the skin’s outer layer, mast cells have numerous receptors that can trigger a spectrum of cellular responses, including rosacea symptoms. The researchers noted that in mice treated with mast cell stabilizers, inflammation did not develop.
In the new study, the researchers will determine whether use of the mast cell stabilizer, topical cromolyn sodium, will decrease symptoms associated with rosacea. In addition, they will study whether levels of 2 enzymes, tryptase and chymotryptase — typically higher in rosacea patients — revert to normal after application of the mast cell stabilizer, and which of the enzymes is more important in this process.
Dr. Yoshikazu Uchida, research dermatologist, and Dr. Peter Elias, professor of dermatology at the University of California-San Francisco, were awarded $25,000 for further study of a biochemical pathway that may lead to inflammation. In recently completed NRS-funded research, Dr. Uchida identified that triggers, such as sunlight and irritated skin, may stress the endoplasmic reticulum, a membrane involved in sorting proteins. In individuals with rosacea, this stress may set off a chain of chemical responses that produce symptoms of the disorder.
In the new research, the investigators will observe the effects of blocking this and other pathways. In addition, the researchers will study whether the application of topical substances known as resveratrol and fatty acid derivative blocks inflammation on skin with rosacea.
The NRS is also continuing to fund ongoing studies by Drs. Meg Gerstenblith and Daniel Popkin at Case Western Reserve University on the incidence of rosacea in fraternal and identical twins; Drs. Ulf Meyer-Hoffert and Thomas Schwartz at the University Clinic Schleswig-Holstein on inhibitors of enzymes that might contribute to the disease process, and Dr. Barbara Summerer at Johns Hopkins University School of Medicine on evaluating specific microbes in rosacea patients.
“We are pleased to provide support for these important new studies, which build upon earlier findings that have added significantly to our knowledge about the causes of rosacea,” explains Dr. Mark Dahl, chairman of the NRS Medical Advisory Board, which selects grant applications for funding. “Thanks to the donations of thousands of individuals with rosacea, ongoing research is now producing a significantly better understanding of the disease process and potential new advances in therapy.”
Researchers interested in applying for grants may obtain forms and instructions through the research grants section of the NRS website. The deadline for submitting proposals to receive a research grant in 2014 is May 7, 2014.n
Valeant to Acquire Solta
Solta manufactures and markets energy-based medical device systems for aesthetic applications, including the Thermage CPT system; the Fraxel repair system; the Clear + Brilliant system and the Liposonix system. Solta had total revenue of approximately $145 million in 2012.
“The acquisition of Solta will bring tremendous value to Valeant’s current aesthetic portfolio and together with our previous acquisitions, will create the broadest aesthetic portfolio in the industry,” says J. Michael Pearson, chairman and chief executive officer of Valeant. “Solta’s leading aesthetic devices are a natural fit with Valeant’s facial injectables, professional skincare products and physician dispensed products and will establish Valeant in a strong leadership position as we continue to build our presence in the aesthetic market. Moreover, this transaction will further enhance our ability to offer dermatologists and plastic surgeons the most comprehensive aesthetic product offering.”
Isotretinoin Patient Assistance Program Launches
Promius Pharma has introduced the Promius Promise Patient Assistance Program for eligible patients who are uninsured, or whose insurance company does not provide coverage for isotretinoin and who otherwise meet program eligibility requirements. In April 2013, Promius introduced isotretinoin capsules USP (Zenatane).
“Severe acne is a disease that can have a profound impact and an isotretinoin patient assistance program is sorely needed. I appreciate the effort that Promius Pharma has put in to make this happen,” says Guy Webster, MD, dermatologist and past president of The American Acne & Rosacea Society, and member of American Academy of Dermatology Retinoids Task Force.
This new patient assistance program will be integrated into the Promius Promise Program, which is designed to help patients and healthcare providers meet treatment requirements. More than 5,000 patients are being supported by the Promius Promise program.
The Promius Promise Patient Assistance Program will potentially help patients find alternative insurance coverage. If this is not possible, the medication will be provided at no cost to the eligible patient through the program. The Promius Promise Patient Assistance Program will assess patient eligibility for alternate health insurance/funding sources based on patient demographic and financial information and drug coverage information. This information is contained in a proprietary alternate payer database that includes such potential payers as Medicaid, the State Children’s Health Insurance Programs and state prescription drug assistance programs, among others. Program materials will provide a summary of eligibility requirements and other restrictions.
To help initiate this program, Promius Pharma has teamed up with a leading non-profit global health organization, AmeriCares.
AAD Releases Position Statement on BCC and SCC Treatment Options
The American Academy of Dermatology (AAD) recently released a position paper on superficial radiation therapy and electronic surface brachytherapy. The paper explains the technology and offers dermatologists 10 guiding principles regarding provision of superficial radiation therapy and electronic surface brachytherapy services “in order to provide high quality care for patients, but is not intended to establish a legal or medical standard of care,” according to the Academy.
The Academy notes that surgical treatment (eg, excision, Mohs, destruction) is the optimal primary intervention for basal cell carcinomas (BCC) and squamous cell carcinomas (SCC) and notes that superficial radiation therapy is a consideration as a second-line option for the treatment of BCC and SCC, for use in special circumstances, including when surgical intervention is contraindicated and after the benefits and risks of treatment alternatives have been discussed with the patient.
In addition, the Academy advises that more research is needed on superficial radiation therapy and electronic surface brachytherapy. It also notes that dermatologists providing superficial radiation therapy must have adequate education and training to administer the therapies. (Electronic surface brachytherapy should not be administered by a dermatologist, according to the current manufacturer’s recommendations.)
The statement also cautions dermatologists to be aware that “superficial radiation therapy and/or electronic surface brachytherapy devices are being marketed to dermatologists as technologically advanced devices with significant current and future revenue streams” and that the Academy’s Code of Ethics for Dermatologists “precludes patient management based on business models designed solely for the financial gain to the dermatology practice, without adequate concern for the best interests of the patient. Such an approach would undermine quality of care and compromise patient safety, and could subject the practice to ethical scrutiny.”
The Academy adds that, “It is important that any practice using superficial radiation therapy, electronic brachytherapy or similar therapies expend appropriate efforts to understand and use proper CPT coding for the service that is verified as such by an authoritative entity other than the device manufacturer.”
Please visit https://aad.org/ to read the position statement in its entirety.
Mechlorethamine gel 0.016% now available in the US
Valchlor (mechlorethamine, Actelion), the first FDA-approved topical formulation of mechlorethamine, is now available for patients in the United States. The gel, which is applied topically once a day, is an alkylating drug indicated to treat patients with stage IA and IB mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL) who have received prior skin-directed therapy. It is an orphan drug that was acquired by Actelion US Holding Company, a subsidiary of Actelion Ltd., in September 2013 as part of a merger with Ceptaris Therapeutics, Inc. The gel is distributed in the US by Accredo Specialty Pharmacy.
“The availability of Valchlor is exciting news for patients and the treatment community. Physicians now have the option to treat appropriate MF-CTCL patients with the first formulation of topical mechlorethamine that is FDA approved based on rigorous clinical evidence to support its use,” says Youn H. Kim, MD, Joanne and Peter Haas Jr. professor for cutaneous lymphoma research, professor of dermatology, and director, Multidisciplinary Cutaneous Lymphoma Clinic, Stanford University School of Medicine.
“The use of topical mechlorethamine has been documented over several decades,” explains Stuart Lessin, MD, former director of dermatology at the Fox Chase Cancer Center, president of the Board of Directors of the Cutaneous Lymphoma Foundation and lead investigator in the pivotal trial. “With the launch of Valchlor, physicians can prescribe with confidence knowing that an FDA-approved formulation is now available, along with support and financial-assistance programs for eligible patients to help facilitate education and access.”
Mycosis fungoides, the most common type of cutaneous T-cell lymphoma, is a rare form of non-Hodgkin’s lymphoma. In the Unite States, approximately 20,000 patients are diagnosed with stage IA-IB MF-CTCL, qualifying it as a rare or orphan disease.
Nominations Being Accepted for Practice Manager of the Year Award
 The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
A committee of ADAM members will review nominations to determine the inaugural recipient. The winner will receive a cash prize of $1,000, and a registration scholarship to attend the 2015 ADAM Annual Meeting. The recipient of the award will be announced at the 2014 ADAM Annual Meeting, held in Denver, Colorado, March 19-21, 2014.
“The Practice Manager of the Year recognizes those individuals who exceed all standards within the profession. We are grateful to CareCredit for its support in establishing and supporting this endeavor,” says Pam Kroussakis, executive director, the ADAM.
Article continues on page 2
{{pagebreak}}
NRS Awards New Grants for Rosacea Research
 The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
Dr. Anna Di Nardo, associate professor of medicine at the University of California-San Diego, and colleagues were awarded $25,000 to study whether blocking the activity of mast cells in individuals with rosacea may alleviate inflammation. In an NRS-funded study completed earlier this year, Dr. Di Nardo’s group discovered that mast cells, located at the interface between the nervous system and the vascular system, are the “missing link” between rosacea triggers and inflammation. Positioned just below the skin’s outer layer, mast cells have numerous receptors that can trigger a spectrum of cellular responses, including rosacea symptoms. The researchers noted that in mice treated with mast cell stabilizers, inflammation did not develop.
In the new study, the researchers will determine whether use of the mast cell stabilizer, topical cromolyn sodium, will decrease symptoms associated with rosacea. In addition, they will study whether levels of 2 enzymes, tryptase and chymotryptase — typically higher in rosacea patients — revert to normal after application of the mast cell stabilizer, and which of the enzymes is more important in this process.
Dr. Yoshikazu Uchida, research dermatologist, and Dr. Peter Elias, professor of dermatology at the University of California-San Francisco, were awarded $25,000 for further study of a biochemical pathway that may lead to inflammation. In recently completed NRS-funded research, Dr. Uchida identified that triggers, such as sunlight and irritated skin, may stress the endoplasmic reticulum, a membrane involved in sorting proteins. In individuals with rosacea, this stress may set off a chain of chemical responses that produce symptoms of the disorder.
In the new research, the investigators will observe the effects of blocking this and other pathways. In addition, the researchers will study whether the application of topical substances known as resveratrol and fatty acid derivative blocks inflammation on skin with rosacea.
The NRS is also continuing to fund ongoing studies by Drs. Meg Gerstenblith and Daniel Popkin at Case Western Reserve University on the incidence of rosacea in fraternal and identical twins; Drs. Ulf Meyer-Hoffert and Thomas Schwartz at the University Clinic Schleswig-Holstein on inhibitors of enzymes that might contribute to the disease process, and Dr. Barbara Summerer at Johns Hopkins University School of Medicine on evaluating specific microbes in rosacea patients.
“We are pleased to provide support for these important new studies, which build upon earlier findings that have added significantly to our knowledge about the causes of rosacea,” explains Dr. Mark Dahl, chairman of the NRS Medical Advisory Board, which selects grant applications for funding. “Thanks to the donations of thousands of individuals with rosacea, ongoing research is now producing a significantly better understanding of the disease process and potential new advances in therapy.”
Researchers interested in applying for grants may obtain forms and instructions through the research grants section of the NRS website. The deadline for submitting proposals to receive a research grant in 2014 is May 7, 2014.n
Valeant to Acquire Solta
Solta manufactures and markets energy-based medical device systems for aesthetic applications, including the Thermage CPT system; the Fraxel repair system; the Clear + Brilliant system and the Liposonix system. Solta had total revenue of approximately $145 million in 2012.
“The acquisition of Solta will bring tremendous value to Valeant’s current aesthetic portfolio and together with our previous acquisitions, will create the broadest aesthetic portfolio in the industry,” says J. Michael Pearson, chairman and chief executive officer of Valeant. “Solta’s leading aesthetic devices are a natural fit with Valeant’s facial injectables, professional skincare products and physician dispensed products and will establish Valeant in a strong leadership position as we continue to build our presence in the aesthetic market. Moreover, this transaction will further enhance our ability to offer dermatologists and plastic surgeons the most comprehensive aesthetic product offering.”
Isotretinoin Patient Assistance Program Launches
Promius Pharma has introduced the Promius Promise Patient Assistance Program for eligible patients who are uninsured, or whose insurance company does not provide coverage for isotretinoin and who otherwise meet program eligibility requirements. In April 2013, Promius introduced isotretinoin capsules USP (Zenatane).
“Severe acne is a disease that can have a profound impact and an isotretinoin patient assistance program is sorely needed. I appreciate the effort that Promius Pharma has put in to make this happen,” says Guy Webster, MD, dermatologist and past president of The American Acne & Rosacea Society, and member of American Academy of Dermatology Retinoids Task Force.
This new patient assistance program will be integrated into the Promius Promise Program, which is designed to help patients and healthcare providers meet treatment requirements. More than 5,000 patients are being supported by the Promius Promise program.
The Promius Promise Patient Assistance Program will potentially help patients find alternative insurance coverage. If this is not possible, the medication will be provided at no cost to the eligible patient through the program. The Promius Promise Patient Assistance Program will assess patient eligibility for alternate health insurance/funding sources based on patient demographic and financial information and drug coverage information. This information is contained in a proprietary alternate payer database that includes such potential payers as Medicaid, the State Children’s Health Insurance Programs and state prescription drug assistance programs, among others. Program materials will provide a summary of eligibility requirements and other restrictions.
To help initiate this program, Promius Pharma has teamed up with a leading non-profit global health organization, AmeriCares.
AAD Releases Position Statement on BCC and SCC Treatment Options
The American Academy of Dermatology (AAD) recently released a position paper on superficial radiation therapy and electronic surface brachytherapy. The paper explains the technology and offers dermatologists 10 guiding principles regarding provision of superficial radiation therapy and electronic surface brachytherapy services “in order to provide high quality care for patients, but is not intended to establish a legal or medical standard of care,” according to the Academy.
The Academy notes that surgical treatment (eg, excision, Mohs, destruction) is the optimal primary intervention for basal cell carcinomas (BCC) and squamous cell carcinomas (SCC) and notes that superficial radiation therapy is a consideration as a second-line option for the treatment of BCC and SCC, for use in special circumstances, including when surgical intervention is contraindicated and after the benefits and risks of treatment alternatives have been discussed with the patient.
In addition, the Academy advises that more research is needed on superficial radiation therapy and electronic surface brachytherapy. It also notes that dermatologists providing superficial radiation therapy must have adequate education and training to administer the therapies. (Electronic surface brachytherapy should not be administered by a dermatologist, according to the current manufacturer’s recommendations.)
The statement also cautions dermatologists to be aware that “superficial radiation therapy and/or electronic surface brachytherapy devices are being marketed to dermatologists as technologically advanced devices with significant current and future revenue streams” and that the Academy’s Code of Ethics for Dermatologists “precludes patient management based on business models designed solely for the financial gain to the dermatology practice, without adequate concern for the best interests of the patient. Such an approach would undermine quality of care and compromise patient safety, and could subject the practice to ethical scrutiny.”
The Academy adds that, “It is important that any practice using superficial radiation therapy, electronic brachytherapy or similar therapies expend appropriate efforts to understand and use proper CPT coding for the service that is verified as such by an authoritative entity other than the device manufacturer.”
Please visit https://aad.org/ to read the position statement in its entirety.
Mechlorethamine gel 0.016% now available in the US
Valchlor (mechlorethamine, Actelion), the first FDA-approved topical formulation of mechlorethamine, is now available for patients in the United States. The gel, which is applied topically once a day, is an alkylating drug indicated to treat patients with stage IA and IB mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL) who have received prior skin-directed therapy. It is an orphan drug that was acquired by Actelion US Holding Company, a subsidiary of Actelion Ltd., in September 2013 as part of a merger with Ceptaris Therapeutics, Inc. The gel is distributed in the US by Accredo Specialty Pharmacy.
“The availability of Valchlor is exciting news for patients and the treatment community. Physicians now have the option to treat appropriate MF-CTCL patients with the first formulation of topical mechlorethamine that is FDA approved based on rigorous clinical evidence to support its use,” says Youn H. Kim, MD, Joanne and Peter Haas Jr. professor for cutaneous lymphoma research, professor of dermatology, and director, Multidisciplinary Cutaneous Lymphoma Clinic, Stanford University School of Medicine.
“The use of topical mechlorethamine has been documented over several decades,” explains Stuart Lessin, MD, former director of dermatology at the Fox Chase Cancer Center, president of the Board of Directors of the Cutaneous Lymphoma Foundation and lead investigator in the pivotal trial. “With the launch of Valchlor, physicians can prescribe with confidence knowing that an FDA-approved formulation is now available, along with support and financial-assistance programs for eligible patients to help facilitate education and access.”
Mycosis fungoides, the most common type of cutaneous T-cell lymphoma, is a rare form of non-Hodgkin’s lymphoma. In the Unite States, approximately 20,000 patients are diagnosed with stage IA-IB MF-CTCL, qualifying it as a rare or orphan disease.
Nominations Being Accepted for Practice Manager of the Year Award
 The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
A committee of ADAM members will review nominations to determine the inaugural recipient. The winner will receive a cash prize of $1,000, and a registration scholarship to attend the 2015 ADAM Annual Meeting. The recipient of the award will be announced at the 2014 ADAM Annual Meeting, held in Denver, Colorado, March 19-21, 2014.
“The Practice Manager of the Year recognizes those individuals who exceed all standards within the profession. We are grateful to CareCredit for its support in establishing and supporting this endeavor,” says Pam Kroussakis, executive director, the ADAM.
Article continues on page 2
{{pagebreak}}
NRS Awards New Grants for Rosacea Research
 The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
Dr. Anna Di Nardo, associate professor of medicine at the University of California-San Diego, and colleagues were awarded $25,000 to study whether blocking the activity of mast cells in individuals with rosacea may alleviate inflammation. In an NRS-funded study completed earlier this year, Dr. Di Nardo’s group discovered that mast cells, located at the interface between the nervous system and the vascular system, are the “missing link” between rosacea triggers and inflammation. Positioned just below the skin’s outer layer, mast cells have numerous receptors that can trigger a spectrum of cellular responses, including rosacea symptoms. The researchers noted that in mice treated with mast cell stabilizers, inflammation did not develop.
In the new study, the researchers will determine whether use of the mast cell stabilizer, topical cromolyn sodium, will decrease symptoms associated with rosacea. In addition, they will study whether levels of 2 enzymes, tryptase and chymotryptase — typically higher in rosacea patients — revert to normal after application of the mast cell stabilizer, and which of the enzymes is more important in this process.
Dr. Yoshikazu Uchida, research dermatologist, and Dr. Peter Elias, professor of dermatology at the University of California-San Francisco, were awarded $25,000 for further study of a biochemical pathway that may lead to inflammation. In recently completed NRS-funded research, Dr. Uchida identified that triggers, such as sunlight and irritated skin, may stress the endoplasmic reticulum, a membrane involved in sorting proteins. In individuals with rosacea, this stress may set off a chain of chemical responses that produce symptoms of the disorder.
In the new research, the investigators will observe the effects of blocking this and other pathways. In addition, the researchers will study whether the application of topical substances known as resveratrol and fatty acid derivative blocks inflammation on skin with rosacea.
The NRS is also continuing to fund ongoing studies by Drs. Meg Gerstenblith and Daniel Popkin at Case Western Reserve University on the incidence of rosacea in fraternal and identical twins; Drs. Ulf Meyer-Hoffert and Thomas Schwartz at the University Clinic Schleswig-Holstein on inhibitors of enzymes that might contribute to the disease process, and Dr. Barbara Summerer at Johns Hopkins University School of Medicine on evaluating specific microbes in rosacea patients.
“We are pleased to provide support for these important new studies, which build upon earlier findings that have added significantly to our knowledge about the causes of rosacea,” explains Dr. Mark Dahl, chairman of the NRS Medical Advisory Board, which selects grant applications for funding. “Thanks to the donations of thousands of individuals with rosacea, ongoing research is now producing a significantly better understanding of the disease process and potential new advances in therapy.”
Researchers interested in applying for grants may obtain forms and instructions through the research grants section of the NRS website. The deadline for submitting proposals to receive a research grant in 2014 is May 7, 2014.n
Valeant to Acquire Solta
Solta manufactures and markets energy-based medical device systems for aesthetic applications, including the Thermage CPT system; the Fraxel repair system; the Clear + Brilliant system and the Liposonix system. Solta had total revenue of approximately $145 million in 2012.
“The acquisition of Solta will bring tremendous value to Valeant’s current aesthetic portfolio and together with our previous acquisitions, will create the broadest aesthetic portfolio in the industry,” says J. Michael Pearson, chairman and chief executive officer of Valeant. “Solta’s leading aesthetic devices are a natural fit with Valeant’s facial injectables, professional skincare products and physician dispensed products and will establish Valeant in a strong leadership position as we continue to build our presence in the aesthetic market. Moreover, this transaction will further enhance our ability to offer dermatologists and plastic surgeons the most comprehensive aesthetic product offering.”
Isotretinoin Patient Assistance Program Launches
Promius Pharma has introduced the Promius Promise Patient Assistance Program for eligible patients who are uninsured, or whose insurance company does not provide coverage for isotretinoin and who otherwise meet program eligibility requirements. In April 2013, Promius introduced isotretinoin capsules USP (Zenatane).
“Severe acne is a disease that can have a profound impact and an isotretinoin patient assistance program is sorely needed. I appreciate the effort that Promius Pharma has put in to make this happen,” says Guy Webster, MD, dermatologist and past president of The American Acne & Rosacea Society, and member of American Academy of Dermatology Retinoids Task Force.
This new patient assistance program will be integrated into the Promius Promise Program, which is designed to help patients and healthcare providers meet treatment requirements. More than 5,000 patients are being supported by the Promius Promise program.
The Promius Promise Patient Assistance Program will potentially help patients find alternative insurance coverage. If this is not possible, the medication will be provided at no cost to the eligible patient through the program. The Promius Promise Patient Assistance Program will assess patient eligibility for alternate health insurance/funding sources based on patient demographic and financial information and drug coverage information. This information is contained in a proprietary alternate payer database that includes such potential payers as Medicaid, the State Children’s Health Insurance Programs and state prescription drug assistance programs, among others. Program materials will provide a summary of eligibility requirements and other restrictions.
To help initiate this program, Promius Pharma has teamed up with a leading non-profit global health organization, AmeriCares.
 The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.  The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.





 The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.
The Association of Dermatology Administrators and Managers (ADAM) in collaboration with CareCredit is accepting nominations for a Practice Manager of the Year Award. The award recognizes office professionals for their leadership qualities, management skills, customer service and going above and beyond expectations within their practice and community. The call for nominations is open through 4 pm EST, Friday, January 24, 2014.  The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.
The National Rosacea Society (NRS) has awarded funding for 2 new studies and continuing support for 5 ongoing studies during the year, as part of its research grants program to increase understanding of the potential causes and other key aspects of rosacea.

















