The oral medication apremilast (Otezla, Celgene Corporation) has been approved to treat adults with active psoriatic arthritis (PsA), the form of arthritis that affects some people with psoriasis. Typically, people develop psoriasis and are later diagnosed with PsA. Joint pain, stiffness and swelling are the main signs and symptoms of PsA. It is the first oral medication to be approved for the indication.
The safety and effectiveness of apremilast, an inhibitor of phosphodieasterase-4, were evaluated in 3 multicenter, randomized, double-blind, placebo-controlled clinical trials — PALACE 1, 2 and 3 — involving 1,493 patients with active PsA. These patients were inadequately controlled by disease-modifying antirheumatic drugs and/or biologics.
Patients treated with apremilast showed improvement in signs and symptoms of PsA, including tender and swollen joints and physical function, compared to placebo. In clinical trials, the most common side effects observed in patients treated with apremilast were diarrhea, nausea and headache.
“Relief of pain and inflammation and improving physical function are important treatment goals for patients with active psoriatic arthritis,” explains Curtis Rosebraugh, MD, MPH, director of the Office of Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research. Apremilast provides a new treatment option for patients with this disease, he adds.
New Leishmaniasis Treatment Cleared
Miltefosine (Impavido, Paladin Therapeutics) received FDA approval for the treatment of leishmaniasis, caused by Leishmania, a parasite that is transmitted to humans through sandfly bites. The disease occurs primarily in people who live in the tropics and subtropics. However, most US patients acquire leishmaniasis overseas.
The oral medicine is approved to treat the 3 main types of leishmaniasis:
• Visceral leishmaniasis (affects internal organs)
• Cutaneous leishmaniasis (affects the skin)
• Mucosal leishmaniasis (affects the nose and throat)
It is intended for patients 12 years of age and older. Miltefosine is the first FDA-approved drug to treat cutaneous or mucosal leishmaniasis. The agent’s safety and efficacy were evaluated in 4 clinical trials. A total of 547 patients received miltefosine and 183 patients received either a comparator drug or a placebo. Results from these trials demonstrated that miltefosine is safe and effective in treating visceral, cutaneous and mucosal leishmaniasis.
The most common side effects identified in clinical trials were nausea, vomiting, diarrhea, headache, decreased appetite, dizziness, abdominal pain, itching, drowsiness and elevated levels of liver enzymes (transaminases) and creatinine. The labeling for miltefosine includes a boxed warning to alert patients and healthcare professionals that the drug can cause fetal harm and therefore should not be given to pregnant women. Healthcare professionals should advise women to use effective contraception during and for 5 months after miltefosine therapy.
The FDA granted miltefosine fast track designation, priority review and orphan product designation. According to the regulatory agency, these designations were granted for numerous reasons. The drug demonstrated the potential to fill an unmet medical need in a serious disease or condition; has the potential to be a significant improvement in safety or effectiveness in the treatment of a serious disease or condition and is intended to treat a rare disease.
With this approval, the manufacturer is awarded a Tropical Disease Priority Review Voucher under a provision included in the FDA Amendments Act of 2007 that aims to encourage development of new drugs and biological products for the prevention and treatment of certain tropical diseases.
Infantile Hemangioma Treatment Gains FDA Approval
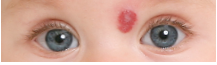 Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
The new drug application for propranolol hydrochloride was submitted to the FDA in May 2013. An application was also submitted to the European Medicines Agency and it received a positive opinion on February 21, 2014 from the Committee for Medicinal Products for Human Use, with marketing authorization expected for April 28, 2014, according to Pierre Fabre Dermatologie.
Propranolol hydrochloride was studied in infants 5 weeks to 5 months old (at therapy initiation) with a proliferative infantile hemangioma requiring systemic treatment in a randomized, double-blind, placebo-controlled, multidose and multicenter adaptive Phase II/III trial, which compared 4 propranolol treatment protocols (1 or 3 mg/kg/day for 3 or 6 months) versus placebo. The treatment protocol of 3 mg/kg/day dose for the duration of 6 months had a 60.4% success rate versus 3.6% in the placebo group (P<0.0001) reaching the primary endpoint: complete or nearly-complete resolution of the target hemangioma. After stopping treatment, 11.4% of patients needed to be retreated.
FDA Green-Lights Chronic Idiopathic Urticaria Treatment
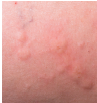 Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab, which is jointly developed by Genentech and Novartis Pharma AG, is the first biologic medicine and first medicine approved by the FDA for CIU since non-sedating H1-antihistamines.
“Chronic idiopathic urticaria can be difficult to manage because its causes are unknown, and other approved medicines aren’t effective enough for many patients,” says Sandra Horning, MD, chief medical officer and head of Global Product Development. “We are pleased to have Xolair as a new option for people with this serious skin condition.”
The efficacy and safety profile of omalizumab for the treatment of CIU was evaluated in 2 clinical studies — ASTERIA I and ASTERIA II. In these studies, patients 12 to 75 years old received doses of omalizumab at 150 mg, 300 mg or placebo. Omalizumab or placebo was given every 4 weeks for 24 weeks (ASTERIA I) and 12 weeks (ASTERIA II). In addition, patients continued to receive H1-antihistamine medicines they had been taking for CIU before starting treatment with omalizumab.
The most common side effects of omalizumab were pain especially in the arms and legs, dizziness, feeling tired, skin rash, bone fractures and pain or discomfort of the ears (in people with allergic asthma), and nausea, headaches, swelling of the inside of the nose, throat or sinuses, cough, joint pain and upper respiratory tract infection (in people with chronic idiopathic urticarial.)
For more news and trends, visit www. the-dermatologist.com/derm-news-wire.
The oral medication apremilast (Otezla, Celgene Corporation) has been approved to treat adults with active psoriatic arthritis (PsA), the form of arthritis that affects some people with psoriasis. Typically, people develop psoriasis and are later diagnosed with PsA. Joint pain, stiffness and swelling are the main signs and symptoms of PsA. It is the first oral medication to be approved for the indication.
The safety and effectiveness of apremilast, an inhibitor of phosphodieasterase-4, were evaluated in 3 multicenter, randomized, double-blind, placebo-controlled clinical trials — PALACE 1, 2 and 3 — involving 1,493 patients with active PsA. These patients were inadequately controlled by disease-modifying antirheumatic drugs and/or biologics.
Patients treated with apremilast showed improvement in signs and symptoms of PsA, including tender and swollen joints and physical function, compared to placebo. In clinical trials, the most common side effects observed in patients treated with apremilast were diarrhea, nausea and headache.
“Relief of pain and inflammation and improving physical function are important treatment goals for patients with active psoriatic arthritis,” explains Curtis Rosebraugh, MD, MPH, director of the Office of Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research. Apremilast provides a new treatment option for patients with this disease, he adds.
New Leishmaniasis Treatment Cleared
Miltefosine (Impavido, Paladin Therapeutics) received FDA approval for the treatment of leishmaniasis, caused by Leishmania, a parasite that is transmitted to humans through sandfly bites. The disease occurs primarily in people who live in the tropics and subtropics. However, most US patients acquire leishmaniasis overseas.
The oral medicine is approved to treat the 3 main types of leishmaniasis:
• Visceral leishmaniasis (affects internal organs)
• Cutaneous leishmaniasis (affects the skin)
• Mucosal leishmaniasis (affects the nose and throat)
It is intended for patients 12 years of age and older. Miltefosine is the first FDA-approved drug to treat cutaneous or mucosal leishmaniasis. The agent’s safety and efficacy were evaluated in 4 clinical trials. A total of 547 patients received miltefosine and 183 patients received either a comparator drug or a placebo. Results from these trials demonstrated that miltefosine is safe and effective in treating visceral, cutaneous and mucosal leishmaniasis.
The most common side effects identified in clinical trials were nausea, vomiting, diarrhea, headache, decreased appetite, dizziness, abdominal pain, itching, drowsiness and elevated levels of liver enzymes (transaminases) and creatinine. The labeling for miltefosine includes a boxed warning to alert patients and healthcare professionals that the drug can cause fetal harm and therefore should not be given to pregnant women. Healthcare professionals should advise women to use effective contraception during and for 5 months after miltefosine therapy.
The FDA granted miltefosine fast track designation, priority review and orphan product designation. According to the regulatory agency, these designations were granted for numerous reasons. The drug demonstrated the potential to fill an unmet medical need in a serious disease or condition; has the potential to be a significant improvement in safety or effectiveness in the treatment of a serious disease or condition and is intended to treat a rare disease.
With this approval, the manufacturer is awarded a Tropical Disease Priority Review Voucher under a provision included in the FDA Amendments Act of 2007 that aims to encourage development of new drugs and biological products for the prevention and treatment of certain tropical diseases.
Infantile Hemangioma Treatment Gains FDA Approval
 Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
The new drug application for propranolol hydrochloride was submitted to the FDA in May 2013. An application was also submitted to the European Medicines Agency and it received a positive opinion on February 21, 2014 from the Committee for Medicinal Products for Human Use, with marketing authorization expected for April 28, 2014, according to Pierre Fabre Dermatologie.
Propranolol hydrochloride was studied in infants 5 weeks to 5 months old (at therapy initiation) with a proliferative infantile hemangioma requiring systemic treatment in a randomized, double-blind, placebo-controlled, multidose and multicenter adaptive Phase II/III trial, which compared 4 propranolol treatment protocols (1 or 3 mg/kg/day for 3 or 6 months) versus placebo. The treatment protocol of 3 mg/kg/day dose for the duration of 6 months had a 60.4% success rate versus 3.6% in the placebo group (P<0.0001) reaching the primary endpoint: complete or nearly-complete resolution of the target hemangioma. After stopping treatment, 11.4% of patients needed to be retreated.
FDA Green-Lights Chronic Idiopathic Urticaria Treatment
 Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab, which is jointly developed by Genentech and Novartis Pharma AG, is the first biologic medicine and first medicine approved by the FDA for CIU since non-sedating H1-antihistamines.
“Chronic idiopathic urticaria can be difficult to manage because its causes are unknown, and other approved medicines aren’t effective enough for many patients,” says Sandra Horning, MD, chief medical officer and head of Global Product Development. “We are pleased to have Xolair as a new option for people with this serious skin condition.”
The efficacy and safety profile of omalizumab for the treatment of CIU was evaluated in 2 clinical studies — ASTERIA I and ASTERIA II. In these studies, patients 12 to 75 years old received doses of omalizumab at 150 mg, 300 mg or placebo. Omalizumab or placebo was given every 4 weeks for 24 weeks (ASTERIA I) and 12 weeks (ASTERIA II). In addition, patients continued to receive H1-antihistamine medicines they had been taking for CIU before starting treatment with omalizumab.
The most common side effects of omalizumab were pain especially in the arms and legs, dizziness, feeling tired, skin rash, bone fractures and pain or discomfort of the ears (in people with allergic asthma), and nausea, headaches, swelling of the inside of the nose, throat or sinuses, cough, joint pain and upper respiratory tract infection (in people with chronic idiopathic urticarial.)
For more news and trends, visit www. the-dermatologist.com/derm-news-wire.
The oral medication apremilast (Otezla, Celgene Corporation) has been approved to treat adults with active psoriatic arthritis (PsA), the form of arthritis that affects some people with psoriasis. Typically, people develop psoriasis and are later diagnosed with PsA. Joint pain, stiffness and swelling are the main signs and symptoms of PsA. It is the first oral medication to be approved for the indication.
The safety and effectiveness of apremilast, an inhibitor of phosphodieasterase-4, were evaluated in 3 multicenter, randomized, double-blind, placebo-controlled clinical trials — PALACE 1, 2 and 3 — involving 1,493 patients with active PsA. These patients were inadequately controlled by disease-modifying antirheumatic drugs and/or biologics.
Patients treated with apremilast showed improvement in signs and symptoms of PsA, including tender and swollen joints and physical function, compared to placebo. In clinical trials, the most common side effects observed in patients treated with apremilast were diarrhea, nausea and headache.
“Relief of pain and inflammation and improving physical function are important treatment goals for patients with active psoriatic arthritis,” explains Curtis Rosebraugh, MD, MPH, director of the Office of Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research. Apremilast provides a new treatment option for patients with this disease, he adds.
New Leishmaniasis Treatment Cleared
Miltefosine (Impavido, Paladin Therapeutics) received FDA approval for the treatment of leishmaniasis, caused by Leishmania, a parasite that is transmitted to humans through sandfly bites. The disease occurs primarily in people who live in the tropics and subtropics. However, most US patients acquire leishmaniasis overseas.
The oral medicine is approved to treat the 3 main types of leishmaniasis:
• Visceral leishmaniasis (affects internal organs)
• Cutaneous leishmaniasis (affects the skin)
• Mucosal leishmaniasis (affects the nose and throat)
It is intended for patients 12 years of age and older. Miltefosine is the first FDA-approved drug to treat cutaneous or mucosal leishmaniasis. The agent’s safety and efficacy were evaluated in 4 clinical trials. A total of 547 patients received miltefosine and 183 patients received either a comparator drug or a placebo. Results from these trials demonstrated that miltefosine is safe and effective in treating visceral, cutaneous and mucosal leishmaniasis.
The most common side effects identified in clinical trials were nausea, vomiting, diarrhea, headache, decreased appetite, dizziness, abdominal pain, itching, drowsiness and elevated levels of liver enzymes (transaminases) and creatinine. The labeling for miltefosine includes a boxed warning to alert patients and healthcare professionals that the drug can cause fetal harm and therefore should not be given to pregnant women. Healthcare professionals should advise women to use effective contraception during and for 5 months after miltefosine therapy.
The FDA granted miltefosine fast track designation, priority review and orphan product designation. According to the regulatory agency, these designations were granted for numerous reasons. The drug demonstrated the potential to fill an unmet medical need in a serious disease or condition; has the potential to be a significant improvement in safety or effectiveness in the treatment of a serious disease or condition and is intended to treat a rare disease.
With this approval, the manufacturer is awarded a Tropical Disease Priority Review Voucher under a provision included in the FDA Amendments Act of 2007 that aims to encourage development of new drugs and biological products for the prevention and treatment of certain tropical diseases.
Infantile Hemangioma Treatment Gains FDA Approval
 Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
The new drug application for propranolol hydrochloride was submitted to the FDA in May 2013. An application was also submitted to the European Medicines Agency and it received a positive opinion on February 21, 2014 from the Committee for Medicinal Products for Human Use, with marketing authorization expected for April 28, 2014, according to Pierre Fabre Dermatologie.
Propranolol hydrochloride was studied in infants 5 weeks to 5 months old (at therapy initiation) with a proliferative infantile hemangioma requiring systemic treatment in a randomized, double-blind, placebo-controlled, multidose and multicenter adaptive Phase II/III trial, which compared 4 propranolol treatment protocols (1 or 3 mg/kg/day for 3 or 6 months) versus placebo. The treatment protocol of 3 mg/kg/day dose for the duration of 6 months had a 60.4% success rate versus 3.6% in the placebo group (P<0.0001) reaching the primary endpoint: complete or nearly-complete resolution of the target hemangioma. After stopping treatment, 11.4% of patients needed to be retreated.
FDA Green-Lights Chronic Idiopathic Urticaria Treatment
 Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab, which is jointly developed by Genentech and Novartis Pharma AG, is the first biologic medicine and first medicine approved by the FDA for CIU since non-sedating H1-antihistamines.
“Chronic idiopathic urticaria can be difficult to manage because its causes are unknown, and other approved medicines aren’t effective enough for many patients,” says Sandra Horning, MD, chief medical officer and head of Global Product Development. “We are pleased to have Xolair as a new option for people with this serious skin condition.”
The efficacy and safety profile of omalizumab for the treatment of CIU was evaluated in 2 clinical studies — ASTERIA I and ASTERIA II. In these studies, patients 12 to 75 years old received doses of omalizumab at 150 mg, 300 mg or placebo. Omalizumab or placebo was given every 4 weeks for 24 weeks (ASTERIA I) and 12 weeks (ASTERIA II). In addition, patients continued to receive H1-antihistamine medicines they had been taking for CIU before starting treatment with omalizumab.
The most common side effects of omalizumab were pain especially in the arms and legs, dizziness, feeling tired, skin rash, bone fractures and pain or discomfort of the ears (in people with allergic asthma), and nausea, headaches, swelling of the inside of the nose, throat or sinuses, cough, joint pain and upper respiratory tract infection (in people with chronic idiopathic urticarial.)
For more news and trends, visit www. the-dermatologist.com/derm-news-wire.
 Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company. Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.





 Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company.
Propranolol hydrochloride (Hemangeol, Pierre Fabre Dermatologie) has received FDA approval for the treatment of “proliferating infantile hemangioma requiring systemic therapy.” It is the only drug approved for this indication. Propranolol hydrochloride, an oral solution developed for use in children, will be available June 2014, according to the company. Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
Omalizumab (Xolair, Novartis) received FDA approval for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. The new indication is for subcutaneous use in people (12 years of age and older) who remain symptomatic despite treatment with H1-antihistamine therapy. Until now, H1-antihistamines have been the only approved therapy for CIU, with about 50% of patients having an inadequate response.
















