What Is This Nodular Lesion on the Upper Back?
Case Report

A 75-year-old man with a 3-year history of a slow growing “cyst” on the upper back presented to his dermatologist because the lesion has become painful. The patient denied any history of local trauma. The rest of his medical and surgical histories were noncontributory. On skin examination, there was a 3.2- × 2.2-cm, somewhat circumscribed, indurated, and deep-seated nodule with a central pearly smooth-surfaced erythematous protruded papule with arborizing telangiectasia on the paraspinal right upper back (Figures 1, 2). A clinical diagnosis of an infiltrative keratinocyte neoplasm was suspected, and a deep tangential biopsy was performed.
What Is The Diagnosis?
Keep scrolling for the answer!
Diagnosis:
Superficial Leiomyosarcoma
Superficial leiomyosarcomas are primary leiomyosarcomas arising from the skin. It is a rare neoplasm of smooth muscle of unknown etiology1 representing approximately 3% to 6.5% of soft tissue sarcomas2-4 and make up 7% to 10% of all leiomyosarcomas.5,6 Most superficial leiomyosarcomas are sporadic tumors with mutations in the TP53 or CDKN2A genes.7,8 Most lesions arise de novo, but leiomyosarcomas have been reported to arise from benign leiomyomas, scars, and even nevus sebaceous.9-11 They have also been observed in patients with Reed syndrome,12 Li Fraumeni syndrome,13 and HIV with Epstein-Barr virus reactivation.14,15
Superficial leiomyosarcomas are subcategorized into cutaneous and subcutaneous lesions. Cutaneous leiomyosarcoma are derived from arrector pili muscles and originated in the dermis with extension into the subcutaneous tissue, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscles of adipose tissue and tend to be larger and more aggressive.1,16 Subcutaneous leiomyosarcomas are more likely to present on the extremities compared with cutaneous lesions (62% vs 33%), which are more commonly found on the head and neck.17-20 While the metastatic potential of superficial leiomyosarcomas is low, these tumors demonstrate aggressive growth and local destruction. Additionally, there is a 25% to 37% recurrence rate for superficial leiomyosarcomas after treatment and close follow up is recommended.
Clinical Presentation and Histopathology
Superficial leiomyosarcomas are often misdiagnosed. They most commonly present as skin-colored nodules with nonspecific features, leading to a wide range of differential diagnoses including fibromas, neurofibromas, dermatofibromas, lipomas, intradermal nevi, and cysts.22 Recently, dermoscopy has been used to evaluate superficial leiomyosarcomas. However, superficial leiomyosarcoma can mimic common cutaneous tumors such as basal cell carcinomas, squamous cell carcinomas, dermatofibromas, and melanomas even with dermoscopic evaluation.4,22 The most common vascular patterns on dermoscopy are linear, irregular, arborizing, or polymorphic.3 Occasionally, white structures, milky red areas, ulceration, and even pigment networks can also be appreciated.3, 23-25
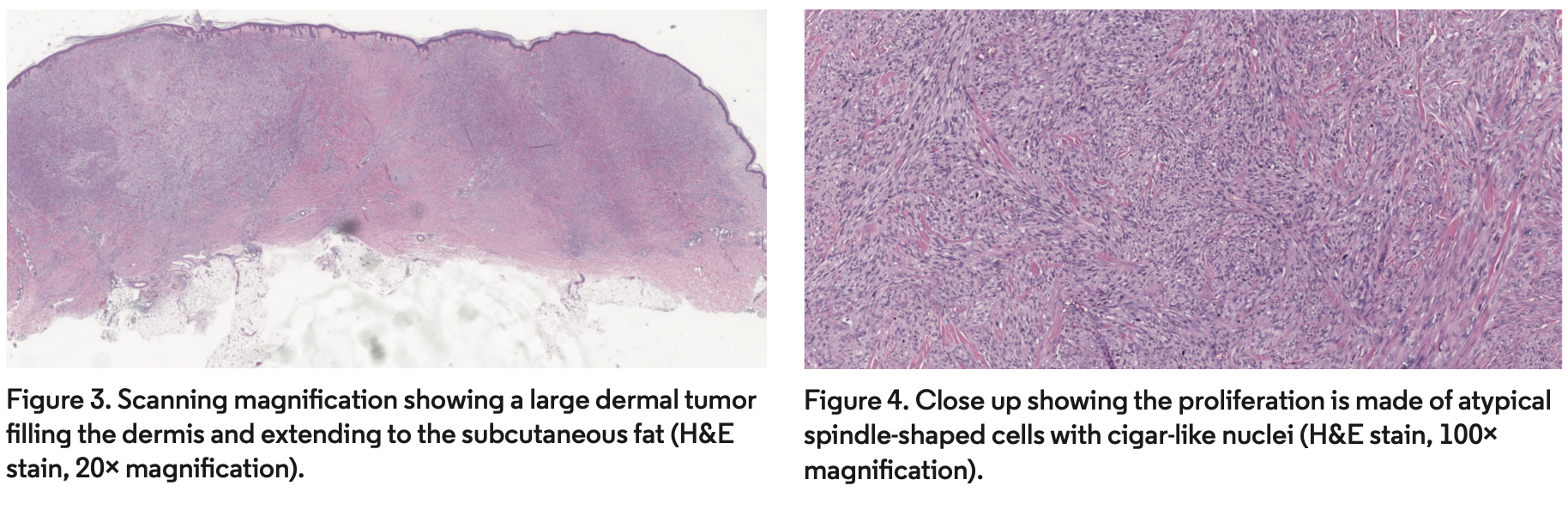
Diagnosis requires histopathologic evaluation. Atypical spindle-shaped cells with cigar-like nuclei can be observed in fascicles on hematoxylin-eosin (H&E) stain. The most common growth patterns of superficial leiomyosarcomas are nodular and diffuse. The nodular pattern is characterized by high cellularity, more mitosis, and necrosis whereas the diffuse pattern is characterized by low cellularity, fewer mitosis, and lack of necrosis.26 Smooth muscle actin and vimentin stains are positive in most cases, but desmin is only expressed in about 60% of superficial leiomyosarcomas.26
Treatment
Treatment is most often wide local excision, but the recommended margins of 2 cm to 5 cm can pose reconstructive challenges.27 More recently, Mohs micrographic surgery has gained popularity for treatment of leiomyosarcoma due to superior margin control, tissue sparing, and lowering recurrence rates to 2% to 14%.5,28,29
Our Patient
A deep shave biopsy was performed by his primary dermatologist due to concerns for keratinocyte carcinoma. The biopsy sample demonstrated a dermal neoplasm composed of interlacing bundles of apparent smooth muscle fibers intermingled with various amounts of collagen bundles. The spindle-shaped cells were thin with blunted edges and long nuclei, some with subnuclear vacuoles, some with enlarged and hyperchromatic nuclei, and arranged in intersecting fascicles. A few mitotic figures were observed on all sections examined. The overlying epidermis showed mild acanthosis (Figures 3, 4). The spindle-shaped cells expressed smooth muscle actin. There was also some weak staining of desmin focally and Ki67 in scattered cells. Staining for S100, SOX10, CD68, AE1/3, and CK903 were negative.
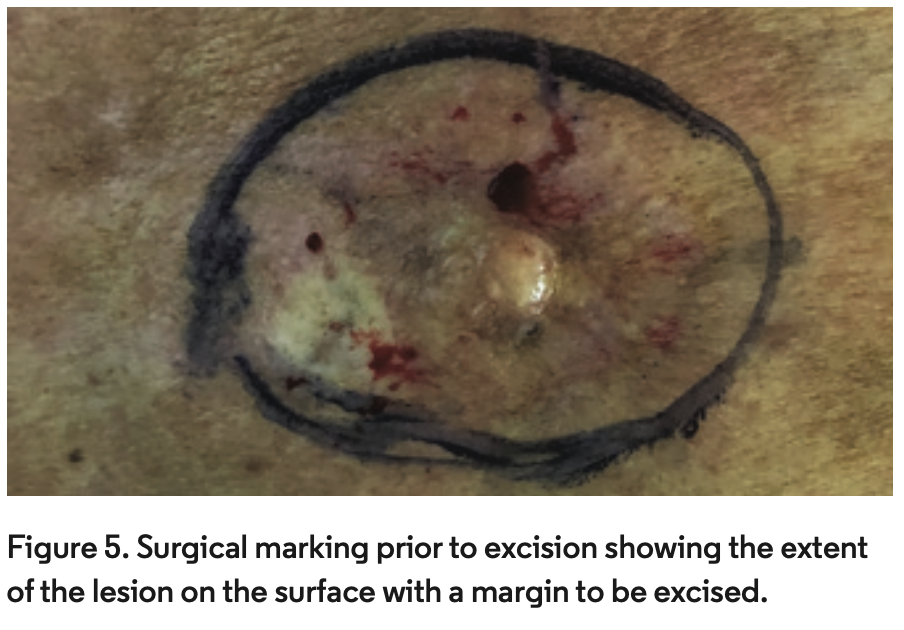
The patient underwent wide local excision with a 3-cm margin outside of the infiltrative area and deep to the fascia with complete clearance of the tumor (Figure 5).
Conclusion
Superficial leiomyosarcoma is a rare tumor of smooth muscle origin that is difficult to diagnose due to similarities in its clinical and dermoscopic presentations as other cutaneous neoplasms. Diagnosis often requires clinicohistopathological correlation and staining is strongly recommended with high degree of suspicion. The standard treatment is wide local excision with a 2- to 5-cm margin, but Mohs micrographic surgery is becoming increasingly popular for treatment of superficial leiomyosarcoma.
Acknowledgement
We extend our appreciation to Dr Amir Zahir, who assisted with the history and evaluation of the manuscript.
Dr Ge is a dermatologist in the department of dermatology at University of Maryland Medical Center in Baltimore, MD. Dr Khachemoune is on faculty at the Veterans Affairs Hospital and SUNY Down-state Dermatology Service in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships.
References
1. Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56(2):251-262. doi:10.1111/j.1365-2559.2009.03471.x
2. Fields JP, Helwig EB. Leiomyosarcoma of the skin and subcutaneous tissue. Cancer. 1981;47(1):156-169. doi:10.1002/1097-0142(19810101)47:1<156::aid-cncr2820470127>3.0.co;2-#
3. Zaballos P, Del Pozo LJ, Argenziano G, et al. Dermoscopy of cutaneous smooth muscle neoplasms: a morphological study of 136 cases. J Eur Acad Dermatol Venereol. 2019;33(4):693-699. doi:10.1111/jdv.15392
4. Atzori L, Pilloni L, Zanniello R, Ferreli C, Rongioletti F. Clear-cell variant of superficial cutaneous leiomyosarcoma associated with RB1 mutation: clinical, dermoscopic, and histopathological characteristics. J Cutan Pathol. 2020;47(6):571-575. doi:10.1111/cup.13655
5. Starling J, Coldiron BM. Mohs micrographic surgery for the treatment of cutaneous leiomyosarcoma. J Am Acad Dermatol. 2011;64(6):1119-1122. doi:10.1016/j.jaad.2010.11.051
6. De Giorgi V, Sestini S, Massi D, Papi F, Alfaioli B, Lotti T. Superficial cutaneous leiomyosarcoma: a rare, misleading tumor. Am J Clin Dermatol. 2008;9(3):185-187. doi:10.2165/00128071-200809030-00008
7. Konomoto T, Fukuda T, Hayashi K, Kumazawa J, Tsuneyoshi M. Leiomyosarcoma in soft tissue: examination of p53 status and cell proliferating factors in different locations. Hum Pathol. 1998;29(1):74-81. doi:10.1016/s0046-8177(98)90393-8
8. Kawaguchi K, Oda Y, Saito T, et al. Mechanisms of inactivation of the p16INK4a gene in leiomyosarcoma of soft tissue: decreased p16 expression correlates with promoter methylation and poor prognosis. J Pathol. 2003;201(3):487-495. doi:10.1002/path.1419
9. González-Vela MC, Val-Bernal JF, Rubio S, Olalla JJ, González-López MA. Cutaneous leiomyosarcoma developing on a pacemaker pocket. Dermatol Surg. 2009;35(5):863-867. doi:10.1111/j.1524-4725.2009.01145.x
10. González-Sixto B, De la Torre C, Pardavila R, Carpintero ML. Leiomyosarcoma arising from scrofuloderma scar. Clin Exp Dermatol. 2008;33(6):776-778. doi:10.1111/j.1365-2230.2008.02805.x
11. Pol RA, Dannenberg H, Robertus JL, van Ginkel RJ. Cutaneous leiomyosarcoma arising in a smallpox scar. World J Surg Oncol. 2012;10:148. doi:10.1186/1477-7819-10-148
12. Wang C, Tetzlaff M, Hick R, Duvic M. Reed syndrome presenting with leiomyosarcoma. JAAD Case Rep. 2015;1(3):150-152. doi:10.1016/j.jdcr.2015.02.004
13. Sabater-Marco V, Ferrando-Roca F, Morera-Faet A, García-García JA, Bosch SB, López-Guerrero JA. Primary cutaneous leiomyosarcoma arising in a patient with Li-Fraumeni syndrome: a neoplasm with unusual histopathologic features and loss of heterozygosity at TP53 gene. Am J Dermatopathol. 2018;40(3):225-227. doi:10.1097/DAD.0000000000000919
14. Tetzlaff MT, Nosek C, Kovarik CL. Epstein-Barr virus-associated leiomyosarcoma with cutaneous involvement in an African child with human immunodeficiency virus: a case report and review of the literature. J Cutan Pathol. 2011;38(9):731-739. doi:10.1111/j.1600-0560.2011.01721.x
15. McClain KL, Leach CT, Jenson HB, et al. Association of Epstein-Barr virus with leiomyosarcomas in young people with AIDS. N Engl J Med. 1995;332(1):12-18. doi:10.1056/NEJM199501053320103
16. Bali A, Kangle R, Roy M, Hungund B. Primary cutaneous leiomyosarcoma: a rare malignant neoplasm. Indian Dermatol Online J. 2013;4(3):188-190. doi:10.4103/2229-5178.115513
17. Bernstein SC, Roenigk RK. Leiomyosarcoma of the skin. Treatment of 34 cases. Dermatol Surg. 1996;22(7):631-635. doi:10.1111/j.1524-4725.1996.tb00609.x
18. Annest NM, Grekin SJ, Stone MS, Messingham MJ. Cutaneous leiomyosarcoma: a tumor of the head and neck. Dermatol Surg. 2007;33(5):628-633. doi:10.1111/j.1524-4725.2007.33124.x
19. Ciurea ME, Georgescu CV, Radu CC, Georgescu CC, Stoica LE. Cutaneous leiomyosarcoma - case report. J Med Life. 2014;7(2):270-273.
20. Torres T, Oliveira A, Sanches M, Selores M. Superficial cutaneous leiomyosarcoma of the face: report of three cases. J Dermatol. 2011;38(4):373-376. doi:10.1111/j.1346-8138.2010.01105.x
21. Aneiros-Fernandez J, Antonio Retamero J, Husein-Elahmed H, Ovalle F, Aneiros-Cachaza J. Primary cutaneous and subcutaneous leiomyosarcomas: evolution and prognostic factors. Eur J Dermatol. 2016;26(1):9-12. doi:10.1684/ejd.2015.2681
22. De Giorgi V, Scarfì F, Silvestri F, et al. Cutaneous leiomyosarcoma: a clinical, dermoscopic, pathologic case study. Exp Oncol. 2019;41(1):80-81.
23. Escobar GF, Gazzi S, Bonamigo RR. Arborizing telangiectasias may also be a dermoscopic vascular pattern of cutaneous leiomyosarcoma. Dermatol Surg. 2021;47(9):1290-1291. doi:10.1097/DSS.0000000000003141
24. Lozano Salazar AD, Márquez García A, Ortega Medina I, Ríos-Martín JJ. Dermal leiomyosarcoma at the end of the left eyebrow. Actas Dermosifiliogr. 2014;105(9):879-882. doi:10.1016/j.ad.2014.03.003
25. Ehara Y, Yoshida Y, Shiomi T, Yamamoto O. Superficial leiomyosarcoma histopathologically mimicking dermatofibroma: pitfall in the diagnosis. J Dermatol. 2017;44(3):348-349. doi:10.1111/1346-8138.13502
26. Kaddu S, Beham A, Cerroni L, et al. Cutaneous leiomyosarcoma. Am J Surg Pathol. 1997;21(9):979-987. doi:10.1097/00000478-199709000-00001
27. Humphreys TR, Finkelstein DH, Lee JB. Superficial leiomyosarcoma treated with Mohs micrographic surgery. Dermatol Surg. 2004;30(1):108-112. doi:10.1111/j.1524-4725.2004.30018.x
28. Murphy-Chutorian B, Routt E, Vinelli G, Ciocon D. A systematic review of the treatment of superficial leiomyosarcoma with Mohs micrographic surgery. Dermatol Surg. 2019;45(12):1437-1441. doi:10.1097/DSS.0000000000001992
29. Huether MJ, Zitelli JA, Brodland DG. Mohs micrographic surgery for the treatment of spindle cell tumors of the skin. J Am Acad Dermatol. 2001;44(4):656-659. doi:10.1067/mjd.2001.112381






















