What Caused These Bluish Bumps?
A 16-year-old man was brought to the dermatology clinic by his father for evaluation of several small bluish “bumps” that he first noticed at least 6 months prior on his left wrist (Figure 1). Since then, he had developed similar lesions on his left heel and right calf (Figures 2 and 3). Some of the lesions seem to have grown, and those on the wrist were occasionally tender, especially with activity or exercise. He denied any recent trauma to the areas, although he reported poking his skin with pencils several years prior to presentation. However, he denied poking his skin in the areas of these lesions. The patient had a history of Asperger syndrome and oppositional defiance disorder; he took no medications. He denied ever being on minocycline. Physical examination was notable for a cluster of 2- to 4-mm nontender, nonblanching, blue/purple macules on the volar left wrist, which appeared distinct from the underlying vessels. Two 3 mm similar lesions were present on the right calf, and one 4-mm lesion was present on the lateral left heel. The patient denied any gastrointestinal symptoms. No other family members had similar lesions. A punch biopsy was performed on one of the lesions on the right calf.

What is your diagnosis?
Diagnosis: Glomulovenous Malformation
Glomulovenous malformations (GVMs), formerly known as glomangiomas, are considered a variant of glomus tumor. They are rare and represent about 10% of all glomus tumors.1-7 GVMs may clinically mimic venous malformation, but are histologically distinct. They consist of aggregates of blood vessels lined by glomus cells. They can be familial or sporadic. Familial cases show autosomal dominant inheritance and are caused by a mutation in the glomulin gene, which is present on chromosome 1p21-p22. Several other mutations have been described.4
Clinical Presentation
While solitary glomus tumors are located on the hands and feet, GVMs tend to present on the limbs and trunk (Figures 1-4). They have also been found in extracutaneous locations including the gastrointestinal (GI) tract, mesentery, bone, mediastinum, lungs, trachea, cervix, and vagina.8-10 GVMs can be further classified as disseminated or localized and can either present as nodules or plaques. Plaques are bluish to red in color, without a central depression3; whereas, the small subcutaneous nodular form can be bluish in color or nonpigmented.1 The lesions are usually less than 1 cm.3 Most lesions are nontender, although some larger lesions may be sensitive to temperature and pressure.4 While solitary glomus tumors are most often seen in adults, GVMs usually present in childhood or adolescence.1-7 There is a slight male predominance.3 In about 60% of cases, there is a positive family history of similar lesions.3

Etiology
GVMs tend to present in nonacral skin where glomus bodies are not normally found. It is suggested that they originate from modified smooth muscle cells of glomus bodies.6 They are thought to arise from a congenital defect of the glomulin gene on chromosome 1p21-p22.4 Inheritance is autosomal dominant with incomplete penetrance and variable expressivity.7 A review by Parsons and colleagues3 found that 60% of glomangioma cases were familial. GVMs are thought to be malformations due to their early presentation.10 Potential triggers include trauma and increased estrogen states, such as pregnancy.3 Rare cases of malignant transformation and metastasis of GVMs and true glomus tumors have been reported.10
Diagnosis
The typical histologic presentation includes irregularly-shaped, dilated vascular spaces surrounded by a thin layer of glomus cells in the dermis and subcutaneous layers.6,7 The vessels may be hyalinized and thrombosed, and the glomus cells appear small, cuboidal, and pink with rounded nuclei.3 Immunohistochemical staining may be positive for smooth muscle markers, including alpha smooth muscle actin, h-caldesmon, HHF35 (pan-actin), and calponin.4 A smooth muscle myosin heavy chain immunostain was used to highlight the cells in our case. Histological distinction should be made from the closely related entities. Glomus tumors are more solid compared with GVMs and have less prominent vasculature. Glomangiomyoma is a closely related entity with a prominence of vascular smooth muscle bundles mixed with glomus cells. Venous malformations do not have a prominence of glomus cells. Diagnosis of GVMs may be aided by radiography, ultrasound, and/or magnetic resonance imaging (MRI). MRI is the most sensitive imaging modality for detecting extracutaneous lesions and the extent of the tumor burden.7 On MRI, GVMs show subtle arterial enhancement, early venous shunting, and progressive filling of dilated venous spaces.11
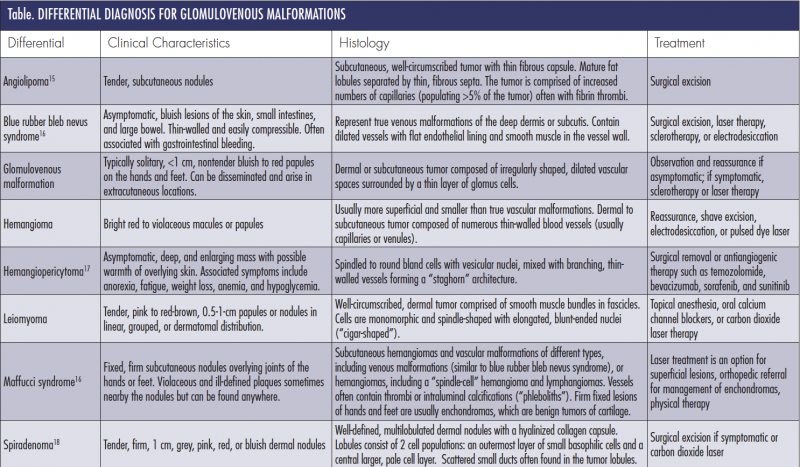
Differential Diagnosis
Differential diagnosis of GVMs include other blue pigmented lesions, painful or tender tumors, and vascular tumors or malformations (Table). Blue rubber bleb nevus syndrome is a familial multivenous malformation disorder that presents with bluish lesions on the skin, small intestine, and large bowel. These nodules are compressible and nontender, can bleed easily, and can present with GI bleeding.6,7 Maffucci syndrome is a rare genetic disorder that presents with multiple enchondromas, superficial and deep hemangioma, and, rarely, hemangioendotheliomas on the fingers and toes.6 Hemangiomas are benign proliferations of endothelial cells that appear within the first few weeks of life and usually regress by 8 to 10 years of age. GVMs may also be mistaken for spiradenomas or benign tumors of the sweat glands, angiolipomas, leiomyoma, or hemangiopericytomas. Venous malformations can be ruled out by color duplex ultrasound.
Treatment
In an asymptomatic patient, observation and reassurance are viable options. Surgical excision is the best treatment for isolated symptomatic GVMs, but not always practical in cases with multiple GVMs, and recurrence is still possible despite complete excision.1 Sclerotherapy with 23.4% sodium chloride solution can be used as a single treatment method or to shrink lesions in preparation for surgical excision.12 Laser therapy for GVMs is gaining popularity, with several case studies demonstrating success. Argon or carbon dioxide laser therapy is preferred for treatment of superficial GVMs, but does not target deeper lesions effectively.3,13 Pulsed dye laser can decrease pain and thickness of lesions, and in one case study was used in conjunction with Nd:YAG laser to effectively treat large widespread GVMs in a 6-month old infant.13,14
Our Patient
Histology of one lesion from the right calf demonstrated ectatic vascular channels surrounded by an innermost lining of endothelial cells and a surrounding rim of slightly larger oval cells in close proximity, consistent with glomus cells. Scattered CD34+ dermal cells were present around the ectatic space, and the condensed small cells within the wall of the cystic lesion were strongly positive for smooth muscle myosin (Figures 5-8). This was consistent with a GVM. Multiple similar lesions were present elsewhere on clinical exam.
Reassurance was given to the patient and his father regarding the benign nature of this condition, and he was referred for treatment with a pulsed dye laser as several of the lesions did cause him discomfort.

Figure 5. (4× objective, hematoxylin-eosin (H&E)-stained slide)
The epidermis is in the upper left hand corner of the field,
and in the mid to lower dermis, there is an ectatic vascular
space with surrounding layers of small, uniform oval cells,
consistent with glomus cells.

Figure 6. (10× objective, H&E-stained slide) Higher power view of the ectatic
vascular channel, with surrounding layers of the uniform oval glomus cells.

Conclusion
GVMs are rare and represent about 10% of all glomus tumors. The condition usually presents in childhood or adolescence. Histopathology of GVMs is characterized by irregularly-shaped, dilated vascular spaces surrounded by a thin layer of glomus cells in the dermis and subcutaneous layers. Various treatment options are available for symptomatic GVMs, including pulsed dye laser.
 Dr Rusk is a PGY-1 resident at The Christ Hospital, in Cincinnati, OH.
Dr Rusk is a PGY-1 resident at The Christ Hospital, in Cincinnati, OH.
Ms Rettig is a physician assistant practicing dermatology at the University of Toledo Medical Center in Toledo, OH.
Dr Arudra is a dermatopathology fellow at MD Anderson Cancer Center, in Houston, TX.
Dr Thomas is a board-certified dermatopathologist at Mercy St Vincent Medical Center in Toledo, OH.
Dr Gottwald is a board-certified dermatologist and division chief at the University of Toledo Medical Center in Toledo, OH.
Disclosure: The authors report no relevant financial relationships.
References
1. Anakwenze OA, Parker WL, Schiefer TK, Inwards CY, Spinner RJ, Amadio PC. Clinical features of multiple glomus tumors. Dermatol Surg. 2008;34(7):884-890.
2. Perry AW, Sosin M, Weissler JM, Chiaffarano JM, Barnard NJ. Multiple glomus tumors presenting as an aesthetic abnormality. Aesthetic Plast Surg. 2015;39(2):236-239.
3. Parsons ME, Russo G, Fucich L, Millikan LE, Kim R. Multiple glomus tumors. Int J Dermatol. 1997;36(12):894-900.
4. Solovan C, Chiticariu E, Beinsan D, Zurac S, Baderca F. Multiple disseminated glomuvenous malformations: do we know enough? Rom J Morphol Embryol. 2012;53(4):1077-1080.
5. Friere M, Rubin B, Lietman S, Sundaram M. Solitary glomus tumor recurring as multiple glomus tumors. Skeletal Radiol. 2012;41(10):1333-1337.
6. Jabir S, Frew Q, Petkar M, Dziewulski P. Multiple glomovenus malformations presenting in a child: follow-up over a period of 8 years. BMJ Case Rep. 2013:2013. doi:10.1136/bcr-2013-200114.
7. Iliescu OA, Benea V, Georgescu SR, Rusu A, Manolache L. Multiple glomus tumors. J Dermatol Case Rep. 2008;2(2):24-27.
8. Barr HS, Galiando J. Pulmonary glomangiomata and hamartoma in tuberous sclerosis. Arch Pathol. 1964;78:287-291.
9. Ruding R, Harmsen AE. Glomus tumor of the stomach: hemangioglomocytoma. Am Surg. 1962;155:221-229.


























