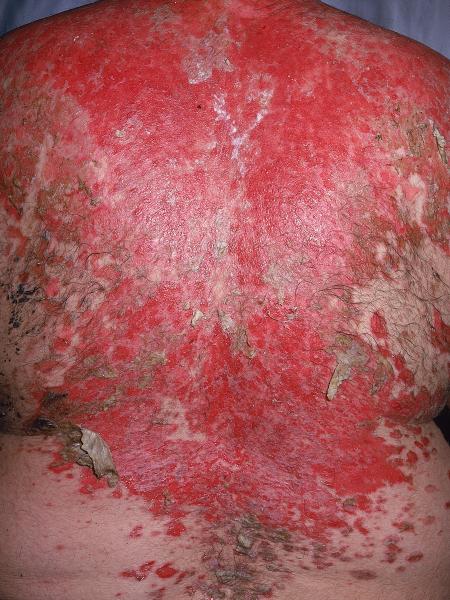
 The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
FDA Breakthrough Therapy Designation is intended to expedite the development and review of medicines with early evidence of potential clinical benefit in serious diseases and to help ensure that patients receive access to medicines as soon as possible. This is the fifteenth Breakthrough Therapy Designation granted to Genentech medicines since 2013. In 2015, the FDA granted Orphan Drug Designation to rituximab for the treatment of pemphigus vulgaris.
Breakthrough Therapy Designation status was granted based on data from the company’s randomized trial conducted in France, which evaluated rituximab plus oral corticosteroid (CS) treatment compared to CS as a first-line treatment in patients with moderate to severe pemphigus. Results of the study, published in The Lancet, show that rituximab may provide substantial improvement in pemphigus vulgaris remission rates and successful tapering and/or cessation of CS therapy.
Photo courtesy: Genentech
 The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
FDA Breakthrough Therapy Designation is intended to expedite the development and review of medicines with early evidence of potential clinical benefit in serious diseases and to help ensure that patients receive access to medicines as soon as possible. This is the fifteenth Breakthrough Therapy Designation granted to Genentech medicines since 2013. In 2015, the FDA granted Orphan Drug Designation to rituximab for the treatment of pemphigus vulgaris.
Breakthrough Therapy Designation status was granted based on data from the company’s randomized trial conducted in France, which evaluated rituximab plus oral corticosteroid (CS) treatment compared to CS as a first-line treatment in patients with moderate to severe pemphigus. Results of the study, published in The Lancet, show that rituximab may provide substantial improvement in pemphigus vulgaris remission rates and successful tapering and/or cessation of CS therapy.
Photo courtesy: Genentech
 The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
FDA Breakthrough Therapy Designation is intended to expedite the development and review of medicines with early evidence of potential clinical benefit in serious diseases and to help ensure that patients receive access to medicines as soon as possible. This is the fifteenth Breakthrough Therapy Designation granted to Genentech medicines since 2013. In 2015, the FDA granted Orphan Drug Designation to rituximab for the treatment of pemphigus vulgaris.
Breakthrough Therapy Designation status was granted based on data from the company’s randomized trial conducted in France, which evaluated rituximab plus oral corticosteroid (CS) treatment compared to CS as a first-line treatment in patients with moderate to severe pemphigus. Results of the study, published in The Lancet, show that rituximab may provide substantial improvement in pemphigus vulgaris remission rates and successful tapering and/or cessation of CS therapy.
Photo courtesy: Genentech
 The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.





 The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.
The FDA has granted rituximab (Rituxan, Genentech) Breakthrough Therapy Designation status for pemphigus vulgaris, a rare, serious and life-threatening condition. The disease is characterized by progressive painful blistering of the skin and mucous membranes. Genentech is enrolling a phase 3 study in pemphigus vulgaris, a disease for which there are limited treatment options.



















