A 71-year-old man presented to the emergency room with significant shortness of breath. Radiographic studies demonstrated hazy bibasilar opacities that were greater in the left lung compared with the right. His low oxygenation necessitated ventilator support on March 27, 2020. Comprehensive radiographic studies of other organ systems did not disclose any abnormalities. The patient was discovered to be SARS-CoV-2 positive based on nasopharyngeal swab assessment. He was then started on hy- droxychloroquine and piperacillin/tazobactam.
The patient’s past medical history was remarkable for hypertension, ulcerative colitis that was well controlled on sulfasalazine, and gastroesophageal reflux disease.

His clinical course was complicated by Escherichia coli and Streptococcus bacteremia and acute kidney failure. The patient was not a candidate for other SARS-CoV-2 therapy due to the concomitant bacteremia. During his intensive care unit hospitalization, the patient developed a distinctive livedoid rash on his hands along with discoloration of his digits suggestive of acral thrombosis (Figure 1). Lesional skin was biopsied along with normal deltoid skin, the latter to assess for systemic complement activation in the event that the patient would require interventional anticomplement therapy. The patient was eventually extubated and transferred to a rehabilitation center, where he developed new symptoms of septic shock and ultimately died from complications.
Light Microscopic Findings
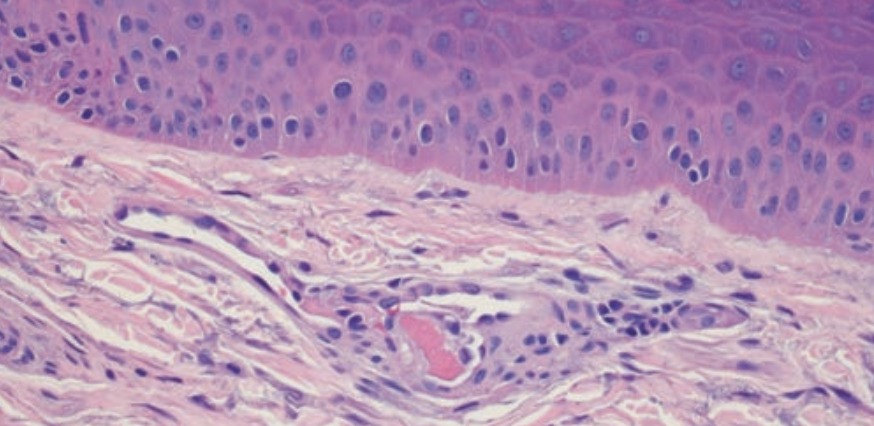
The biopsy of the livedoid rash from the hand demonstrated marked superficial vascular ectasia. In addition, occasional vessels including small arterioles manifested intraluminal fibrin thrombi (Figure 2). The endothelium lining the occluded blood vessels exhibited proplastic alterations. There was no frank vasculitic change as evidenced by the lack of mural fibrin deposition, angiocentric inflammation, and significant red cell extravasation.
Immunohistochemical stains were performed including a myxovirus resistant protein (MxA) stain, the surrogate marker of the type I interferon (IFN) microenvironment, which was found to be negative. Complement studies, namely C3d, C4d, and C5b-9, were conducted on the formalin-fixed paraffin-embedded tissue. The C3d stains exhibited very focal weak staining within the endothelium of
capillaries, venules, and small arterioles within the mid- and deeper dermis. The C4d pattern of endothelial cell staining showed a similar distribution to C3d but with a stronger staining intensity. The C5b-9 studies were very striking with many vessels throughout the dermis exhibiting positive staining (Figures 3 and 4). There was in excess of 45 positive blood vessels per section examined that showed an intense pattern of endothelial and subendothelial staining. Overall, the findings were those of a complement-mediated thrombotic microvascular injury syndrome. The normal deltoid skin biopsy did not show any obvious microvascular thrombosis. There were no clear-cut light microscopic abnormalities that were discernible on routine hematoxylin-eosin (H&E) stained material. Immunohistochemical stains comparable to those conducted on the livedoid skin sample were performed. The MxA stain was negative. The C3d preparation showed endothelial and subendothelial staining of four blood vessels in the mid- and deeper dermis while the C4d demonstrated six positive staining vessels in an endothelial and subendothelial array. The C5b-9 studies exhibited granular microvascular staining within the dermis with 12 positive staining blood vessels. As there were more than 10 vessels exhibiting endothelial and/or subendothelial staining for C5b-9, the deltoid biopsy established a diagnosis of systemic complement activation (Figure 4). Although the patient could be considered a candidate for eculizumab therapy given the general improvement in the patient’s clinical course, therapeutic intervention with eculizumab was not given.
In situ hybridization studies using a highly sensitive assay for SARS-CoV-2 probe were negative. In lesional and nonlesional skin, there was significant expression of SARS-CoV-2 spike glycoprotein and membrane protein within the endothelial cells lining microvessels of the dermis and subcutaneous fat (Figure 5). Additionally, there was enhanced endothelial cell expression of caspase 3 and IL-6.

DISCUSSION
The severe acute respiratory distress syndrome associated with coronavirus 2 (SARS-CoV-2), the etiologic agent of coronavirus disease 2019 (COVID-19), was initially identified in Wuhan, Hubei Province, China, in December 2019. It was documented to be a pandemic by the World Health Organization in early March 2020 and has since been responsible for over 75 million cases worldwide and more than 2.2 million deaths. Organ dysfunction, particularly progressive respiratory failure and a generalized coagulopathy, are associated with the highest mortality.
We demonstrated in an earlier study the important role for mannan-binding lectin pathway complement activation in the pathogenesis of severe and critical COVID-19.6 When one examines fatal cases of COVID-19 of adults, the lung samples exhibit a reproducible morphology, specifically in the context of a pauci-inflammatory necrotizing septal capillary injury syndrome. Prior studies have demonstrated conspicuous deposits of C4d and C5b-9 within the septal microvessels with evidence of colocalization of SARS- CoV-2 associated capsid proteins including spike glycoprotein and membrane and envelope capsid proteins consistent with a virally triggered complement activation syndrome as the mechanism underling COVID-19 associated microangiopathic acute respiratory distress syndrome (ARDS).


It has been demonstrated that in the spike glycoprotein, the critical binding site for the viral receptor, namely angiotensin converting enzyme 2 (ACE-2), has glycosylation sites for high mannose structures that is recognized by certain residues on mannan-binding lectin resulting in MASP-2 activation. The subsequent activation of MASP-2 leads to the formation of C5b-9. MASP-2 is very similar to the C1s molecule, suggesting commonality in molecular origin. The basis of the spike glycoprotein localization to capillary endothelium is attributable to ACE-2 expression in the septal microvessels of the lung. ACE-2 is also found in other microvascular beds such as the skin, explaining the localization of spike glycoprotein and components of complement activation within the skin and other organ systems as will be discussed presently.

























