The coronavirus pandemic has affected every single person since the first lockdown in March 2020, and it has resulted in death, social disconnection, loss of jobs, increased distress, and much, much more. While many people are experiencing a new phenomenon known as COVID fatigue, there is a glimmer of hope with now three vaccines currently being distributed under emergency use authorization (EUA) from the FDA.1 Many are concerned about the rapid rate at which these novel vaccines have been approved; however, mRNA trials have been around since the 1990s with animal testing and have been used in human trials for numerous infections (Zika, rabies, cyto- megalovirus, influenza) and cancer treatment (melanoma, solid organ tumors, lymphomas).2-5
Herein, we will review the most recent efficacy and safety data as well as discuss the impact of mutations in COVID-19 such as the United Kingdom (UK) and South African (SA) variants on vaccine efficacy. We then will discuss some special medical and cosmetic dermatologic patient populations and what precautions should be considered in vaccinating these groups. Please note that this article will largely focus on the mRNA vaccines (mRNA-1273, referred to as the Moderna vaccine, and BNT162b2, referred to as the Pfizer vaccine), as the newer vector vaccine manufactured by Janssen was approved during the time of publication (February 27, 2021).
How Effective is the Vaccine?
After two doses, there is a 95% and 94.5% efficacy rate for the Pfizer and Moderna vaccines respectively.6,7 These rates are very impressive and better than the average annual influenza vaccine as well as the 50% threshold set by the World Health Organization (WHO) for an effective vaccine. To put that into perspective, the annual influenza vaccine has been between 19% to 60% effective in the last decade.7
Immunity to SARS-CoV-2 begins approximately 12 days after the first dose is administered, and two doses are recommended 21 days or 28 days apart for the Pfizer and Moderna vaccines, respectively, to provide a longer-term immune response.7,8
Is the Vaccine Safe?
Vaccine trials take place in stages, starting with trials on animals, and then three trials on humans: phase 1, phase 2, and phase 3. In their phase 3 trials, the Pfizer vaccine included more than 40,000 participants and the Moderna vaccine included more than 30,000 participants.7,8 No safety steps were compromised, and we have 2-month safety data that confirms minor side effects from the phase 3 trials. All study participants will be followed for 2 years for more long-term data.
Posttrial surveillance is also being conducted, and the US government has implemented the most intensive and comprehensive safety monitoring in history with the V-safe, Vaccine Adverse Event Reporting System (VAERS), Clinical Immunization Safety Data Assessment (CISA), and Vaccine Safety Datalink (VSD) programs.9,10 Currently, more than 29.5 million COVID-19 vaccines have been administered in the United States, with the current goal of an additional 150 million doses in the next 100 days.
Vaccine recipients had higher rates of local reactions (pain, erythema, swelling) at the injection site and systemic reactions (fever, headache, myalgias, fatigue) than placebo recipients, with more reactions occurring after the second dose and in younger participants (aged <65 years). Most reactions were mild to moderate and resolved within 1 to 3 days.7,8
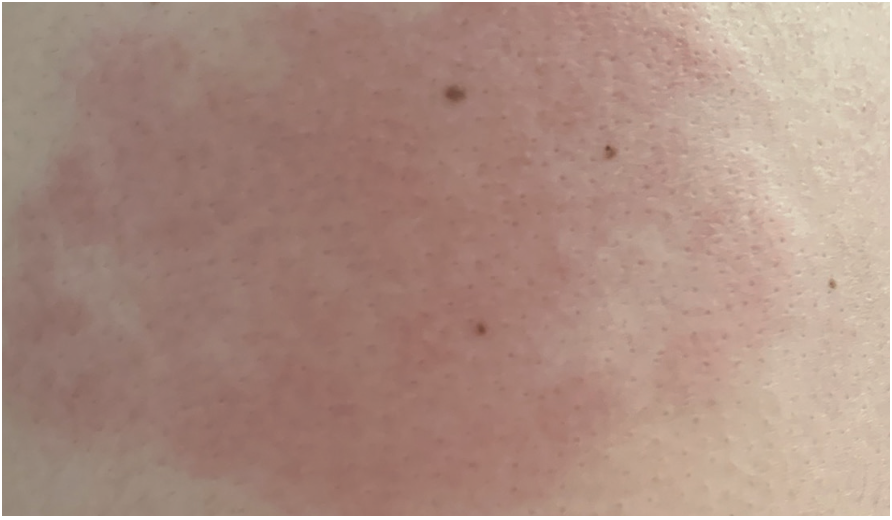
COVID arm is a new delayed skin reaction to the mRNA vaccine that has been recently reported. It most commonly occurs 7 to 10 days after the initial vaccine dose (Figure 1), and the redness, swelling, and itching/pain can occur more rapidly after the second dose (Figure 2). The reaction seems to be more common in women between the ages of 30 to 50 years. People who have this reaction should still receive their second dose of the vaccine. While the reaction can be annoying or concerning for patients, the virus is deadly. Fear of COVID arm is not a reason to avoid getting the vaccine or a second dose. In most cases, no treatment is required. If the rash is itchy, oral antihistamines (eg, loratadine or diphenhydramine) can be beneficial; if the site is tender, acetaminophen can provide symptomatic relief.11 If the rash results in severe itching, pain, or lasts longer than 1 week, patients should seek treatment with their local primary care physician or dermatologist.
A risk of acute hypersensitivity is sometimes observed with vaccines. Anaphylaxis has been reported in both the Pfizer (5.0 cases per million doses) and Moderna (2.8 cases per million doses) vaccines, and it was more common in female patients with a history of allergic reactions or anaphylaxis.12 For comparison, the rate of anaphylaxis of the annual influenza vaccine is 1.3 per million doses given.13
Injectable Filler and COVID-19 mRNA vaccines:

In a FDA report on the Moderna COVID-19 vaccine,14 three cases of swelling that occurred at sites of prior dermal filler placement following vaccination were described. Due to this report and subsequent media coverage, there is an emerging concern among dermatologic patients of the possibility of having localized swelling at areas of dermal hyaluronic acid filler placement after receiving a COVID-19 mRNA vaccine. The official Centers for Disease Control and Prevention (CDC) guidelines state that mRNA COVID-19 vaccines may be administered to persons who have received injectable dermal fillers and that having prior filler is not a contraindication to vaccination.15
The American Society for Dermatologic Surgery (ASDS) also released a statement16 regarding dermal filler reactions as follows: Given currently available data, patients already treated with dermal fillers should not be discouraged or precluded from receiving vaccines of any kind. Similarly, patients who have had vaccines should not be precluded from receiving dermal fillers in the future.
The ASDS also noted that swelling at dermal filler sites is not unique to mRNA vaccination, highlighting that the existing evidence suggest these reactions may be immunologically triggered by viral and bacterial illness, vaccinations such as the influenza vaccine, and dental procedures.16-18 Thankfully, these rare adverse events are temporary and can be managed by an experienced board-certified dermatologist with the use of oral medications or injections to dissolve the filler.
Are There Groups That Should Not Get the Vaccine?
 Currently, the mRNA COVID-19 Pfizer or Moderna vaccines are contraindicated for people with a history of immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including polyethylene glycol [PEG]) or to polysorbate (due to potential cross-reactive hypersensitivity with the vaccine ingredient PEG). People who have recently had COVID-19 should wait 90 days from the time of diagnosis to get the vaccine unless directed differently by their health care provider (HCP).
Currently, the mRNA COVID-19 Pfizer or Moderna vaccines are contraindicated for people with a history of immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including polyethylene glycol [PEG]) or to polysorbate (due to potential cross-reactive hypersensitivity with the vaccine ingredient PEG). People who have recently had COVID-19 should wait 90 days from the time of diagnosis to get the vaccine unless directed differently by their health care provider (HCP).
The vaccines were not studied in children, pregnant women, or patients who are immunocompromised, so there is limited data regarding outcomes in these populations. However, despite it not being studied in patients who are immunocompromised, the vaccine is currently recommended in this patient population. Numerous medical societies, including the CDC and the American College of Obstetricians and Gynecologists, feel that the vaccine should not be withheld from women who are pregnant or breastfeeding. Pregnant patients should decide along with their physician if they should receive the vaccine based on their individual risk of exposure and community COVID-19 rates. Children younger than 16 years should not be vaccinated at this time unless specifically directed by their physician until further clinical trials are performed. 19,20
Treating Psoriatic Disease and Special Considerations for the COVID-19 Vaccine
A task force composed of medical professionals representing the fields of dermatology, rheumatology, and epidemiology along with representation from the National Psoriasis Foundation created guidelines regarding the impact of systemic psoriasis treatments during the COVID-19 pandemic. This task force provided guidance to assist patients with psoriatic disease and their physicians in making strategic and thoughtful decisions regarding their treatment regimens. Existing data does not suggest an increased risk of contracting SARS-CoV-2 infection among patients with psoriasis and/or psoriatic arthritis compared with the general population.21 In fact, biologic therapy for psoriasis may be protective against severe manifestations (intensive care unit [ICU] hospitalization or death) of SARS-CoV-2 infection according to two large international observational studies.22,23
The task force encouraged patients with psoriasis to receive either of the available mRNA-based COVID-19 vaccines once they become readily available to their communities in their tier. Those taking systemic immunomodulating medications, such as corticosteroids, methotrexate, or injectable biologics, to treat their psoriasis and/or psoriatic arthritis can be reassured that these treatments are not contraindications to either of the two mRNA-based COVID-19 vaccines.21 Theoretically, there is risk of a reduced response to vaccination in patients on immunomodulating therapy; however, it is not advised to discontinue systemic psoriasis treatments prior to vaccination as that poses a risk of their symptoms flaring or worsening.
Emerging New Variants of SARS-CoV-2
There are new variants of the coronavirus, raising concerns regarding vaccine efficacy. There is evidence that two of the more infectious new strains originated from SA and UK. The UK variant is estimated to be 50% more transmissible than the current strain.24 While neither of the new strains have proven to be more deadly yet, the increased transmission rates result in a higher rate of infections, reduced ICU and hospital capacity, and higher rates of HCP burnout. This strain on the health care system may ultimately result in increased deaths.25
Pfizer vaccine effectiveness against variant strains. SARS- CoV-2 is characterized by spike proteins, which create its infamous crown-like shape. As previously mentioned, the UK variant is approximately 50% more transmissible than the current strain, and this increase in transmissibility is due to several mutations in the spike protein that distinguish the UK and SA strains from the initial SARS-CoV-2 strain. A particular mutation, known as N501Y, improves the UK variant’s ability to bind to human receptors, thereby promoting its ability to enter human cells and replicate. On January 19, 2021, a study found that the Pfizer COVID-19 vaccine is successful in neutralizing the mutated spike protein of the UK variant.26 Subsequently, an additional study was published on January 27, 2021, that demonstrated that the vaccine is slightly less effective in neutralizing the three spike mutations of the SA variant (E484K+N501Y+D614G).27 However, overall this is great news, as the Pfizer vaccine did promote the production of antibodies that fight off viruses with the mutations present in UK and SA variants. While the vaccine was slightly less effective against the SA strain, Pfizer and its European partner BioNTech “believe the small differences in viral neutralization observed in these studies are unlikely to lead to a significant reduction in the effectiveness of the vaccine.”28 Further monitoring for infections in vaccinated individuals will be necessary to determine the effectiveness of the Pfizer vaccine to prevent severe disease produced by new virus variants, which will ultimately determine if a booster vaccine to these unique strains will be necessary. Thankfully, modifying and adapting the existing mRNA technology to combat new mutations can be done quickly.25
Ms Melnick and Ms Silva are medical assistants at Newport Beach Dermatology and Plastic Surgery in Newport Beach, CA. Dr Lewallen is a dermatologist at Newport Beach Dermatology and Plastic Surgery.
Reference
1. Understanding mRNA COVID-19 Vaccines. Centers for Disease Control and Prevention. Updated December 18, 2020. Accessed February 25, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html
2. Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. doi:10.1016/j.nantod.2019.100766
3. Isaacson W. mRNA technology gave us the first COVID-19 vaccines. It could also upend the drug industry. Time. Published January 11, 2021. Accessed February 25, 2021. https://time.com/5927342/mrna-covid-vaccine/
4. Garde D, Saltzman J. The story of mRNA: From a loose idea to a tool that may help curb Covid. statnews.com. Published November 10, 2020. Accessed February 25, 2021. https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/
5. Moderna’s mRNA Clinical Trials: CMV, MMA, Zika, Several Types of Cancer and Other Diseases. modernatx.com. Accessed February 25, 2021. https://www.modernatx.com/pipeline/modernas-mrna-clinical-trials-cmv-mma-zika-several-types-cancer-and-other-diseases
6. Polack FP, Thomas SJ, Kitchin N, et al; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
7. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
8. CDC seasonal flu vaccine effectiveness studies. cdc.gov. Centers for Disease Control and Prevention. Updated December 11, 2020. Accessed February 25, 2021.https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm
9. V-safe after vaccination health checker. Centers for Disease Control and Prevention. Updated February 6, 2021. Accessed February 25, 2021.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html
10. Ensuring the safety of COVID-19 vaccines in the United States. Centers for Disease Control and Prevention. Updated February 15, 2021. Accessed February 25, 2021.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html
11. Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. Published January 27, 2021. Accessed February 25, 2021. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
12. Lee BY. Moderna Covid-19 vaccine: here is the risk of severe allergic reactions. Forbes Magazine. Published January 23, 2021. Accessed February 25, 2021. https://www.forbes.com/sites/brucelee/2021/01/23/moderna-covid-19-vaccine-here-is-the-risk-of-severe-allergic-reactions/
13. Flu vaccine and people with egg allergies. Centers for Disease Control and Prevention. Updated September 22, 2020. Accessed February 25, 2021.https://www.cdc.gov/flu/prevent/egg-allergies.htm
14. Moderna COVID-19 Vaccine. US Food & Drug Administration; December 17, 2020. Accessed February 25, 2021. https://www.fda.gov/media/144434/download
15. Interim clinical considerations for use of mRNA COVID-19 Vaccines. Centers for Disease Control and Prevention. Updated February 5, 2021. Accessed February 25, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
16. Avram M, Bertucci V, Cox SE, Jones D, Mariwalla K. Guidance regarding SARS-CoV-2 vaccine side effects in dermal filler patients. American Society for Dermatologic Surgery. Published December 28, 2020. Accessed February 25, 2021. https://www.asds.net/Portals/0/PDF/secure/ASDS-SARS-CoV-2-Vaccine-Guidance.pdf
17. Turkmani MG, De Boulle K, Philipp-Dormston WG. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin Cosmet Investig Dermatol. 2019;12(2019):277-283. doi:10.2147/CCID.S198081
18. Bhojani-Lynch T. Late-onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open. 2017;5(12):e1532. doi:10.1097/GOX.0000000000001532
19. Pfizer-BioNTech COVID-19 Vaccine. Centers for Disease Control and Prevention. Updated January 4, 2021. Accessed February 25, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/
20. Moderna COVID-19 Vaccine. Centers for Disease Control and Prevention. Updated December 22, 2020. Accessed February 25, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html
21. Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: Version 1. J Am Acad Dermatol. 2020;83(6):1704-1716. doi:10.1016/j.jaad.2020.09.001
22. Gisondi P, Facheris P, Dapavo P, et al. The impact of the COVID-19 pandemic on patients with chronic plaque psoriasis being treated with biological therapy: the Northern Italy experience. Br J Dermatol. 2020;183(2):373-374. doi:10.1111/bjd.19158
23. Fougerousse A-C, Perrussel M, Bécherel P-A, et al. Systemic or biologic treatment in psoriasis patients does not increase the risk of a severe form of COVID-19. J Eur Acad Dermatol Venereol. 2020;34(11):e676-e679. doi:10.1111/jdv.16761
24. Davies N, Abbott S, Barnard RC, et al; CMMID COVID-19 Working Group. Estimated transmissibility and severity of novel SARS-CoV-2 variant of concern 202012/01 in England. medRxiv. Preprint posted online February 7, 2021. Accessed February 25, 2021. doi:10.1101/2020.12.24.20248822
25. Wu KJ. Here’s what to know about the South African and UK virus variants. The New York Times. Published January 12, 2021. Accessed February 25, 2021. https://www.nytimes.com/2021/01/12/world/covid-19-variant-south-africa-uk.html
26. Muik A, Wallisch A-K, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. bioRxiv. Preprint posted online January 19, 2021. Accessed February 25, 2021. doi:10.1101/2021.01.18.426984
27. Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K, and N501Y variants by BNT162b2 vaccine-elicited sera. bioRxiv. Preprint posted online January 27, 2021. Accessed February 25, 2021. doi:10.1101/2021.01.27.427998
28. Ellis R. Vaccine not as effective against S. African variant. WebMD. Published January 31, 2021. Accessed February 25, 2021. https://www.webmd.com/vaccines/covid-19-vaccine/news/20210131/vaccine-not-as-effective-against-south-african-variant























