FDA Approves Nonprescription Lotion for Treatment of Head Lice
 The FDA announced its latest dermatologic therapy approval of over-the-counter (OTC) ivermectin lotion 0.5% for the treatment of head lice infestation in patients age 6 months and older.
The FDA announced its latest dermatologic therapy approval of over-the-counter (OTC) ivermectin lotion 0.5% for the treatment of head lice infestation in patients age 6 months and older.
Ivermectin lotion, 0.5%, was initially approved as a prescription drug in February 2012. This latest approval follows a prescription-to-OTC switch process, which increases the availability of the lotion. These approval processes are generally initiated by the manufacturer and require data that demonstrate that the drug is safe and effective when used as directed by the consumer without the supervision of a health care professional.
In the United States, an estimated 6 to 12 million cases of head lice infestation occur annually in children aged 3 to 11 years. Infestations are common among child care settings, elementary schools, and households where children have lice.
Reference
FDA approves lotion for nonprescription use to treat head lice. News release. U.S. Food & Drug Administration; October 27, 2020. Accessed October 28, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-lotion-nonprescription-use-treat-head-lice
Contact Dermatitis Caused by Personal Care Products Increasing
Personal care product (PCP)-related contact dermatitis is increasing among men and women, but it differs in the source and location, according to the findings of a recent study published in Journal of the American Academy of Dermatology.
“PCPs are commonly responsible for allergic and irritant contact dermatitis,” the researchers wrote. In the study, they sought to characterize and compare PCP-related contact dermatitis among men and women. Using data from the North American Contact Dermatitis Group (NACDG) from 1996 through 2016, they identified PCPs as a source of irritant contact dermatitis or positive patch test reaction among 4680 of 16,233 men (28.8%) and 12,730 of 32,222 women (39.5%).
The analysis showed that the proportion of PCP-related dermatitis significantly increased over a decade among both men and women.

Compared with women, a larger proportion of men were older and were more likely to have trunk and/or extremity dermatitis caused by PCP, the researchers said. They also found that men were more likely to have soaps as a source of contact dermatitis, while women were twice as likely to have hair care products as a source.
The most common PCP-related NACDG allergens were methylisothiazolinone (male-targeted PCP [MPCP]: 28.8%; female-targeted PCP [FPCP]: 21.5%), fragrance mix I (MPCP: 22.3%; FPCP: 20.1%), balsam of Peru (MPCP: 18.5%; FPCP: 14.1%), quaternium-15 (MPCP: 16.1%: FPCP: 12.3%), and paraphenylenediamine (MPCP: 11.5%; FPCP: 11.1%).
“Sites of involvement and relevant PCP sources are distinct between sexes,” the researchers concluded. “Male and female variation in exposure history may explain differences in reactivity to some allergen groups.”
Reference
Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group (NACDG) Data, 1996-2016. J Am Acad Dermatol. Published online October 8, 2020. doi:10.1016/j.jaad.2020.10.003
Biologics Associated With Lower Risk of COVID-19 Hospitalization
Data from the international registry showed that patients with psoriasis who received a biologic were less likely to be hospitalized vs patients who received a nonbiologic systemic therapy. This data from the PsoProtect registry were published in the Journal of Allergy and Clinical Immunology.
Psoriasis has been previously associated with various diseases considered high risk for severe COVID-19 infection, including cardiovascular disease and metabolic syndrome. The registry was initiated to characterize the course of COVID-19 in patients with psoriasis and to identify factors associated with hospitalization. Clinicians submitted data on a patient with psoriasis with confirmed/suspected COVID-19, and a separate patient-facing registry collected data regarding risk-mitigating behaviors. Multiple logistic regression was used to assess the association between clinical and demographic characteristics and hospitalization rates.
At time of publication, the registry collected 374 clinician-reported patient outcomes from 25 countries. Of these patients, 71%, 18%, and 10% were receiving a biologic therapy, nonbiologic systemic therapy, and no systemic treatment, respectively. In regard to COVID-19 outcomes, 348 (93%) patients fully recovered, 77 (21%) patients were hospitalized, and nine (2%) patients died.
On analysis, increased hospitalization risk was associated with older age, male sex, non-White ethnicity, and comorbid lung disease, similarly to the established risk factors for the general, nonpsoriasis population. However, hospitalization was more frequently reported in patients receiving nonbiologic systemic therapies than those on biologics (odds ratio, 2.84; 95% CI, 1.31-6.18). Further, no significant differences were found between the different biologic classes.
“Biologic use was associated with lower risk of COVID-19-related hospitalization than non-biologic systemic therapies,” stated the research group. “However, further investigation is warranted due to potential selection bias and unmeasured confounding. Established risk factors were associated with higher hospitalization rates.”
Reference
Mahil SK, Dand N, Mason KJ, et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis – insights from a global registry-based study. J Allergy Clin Immunol. Published online October 16, 2020. doi:10.1016/j.jaci.2020.10.007
Long-Hauler Patients Show Prolonged Cutaneous Symptoms of COVID-19
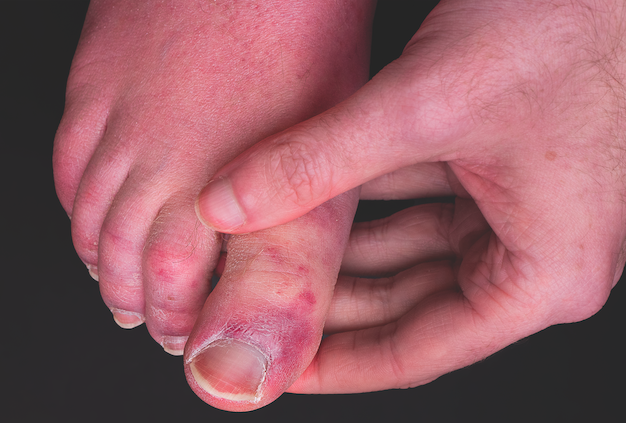 Analysis from the COVID-19 Dermatology Registry revealed that some patients who develop cutaneous symptoms of the disease become “long haulers” with persistent skin symptoms lasting as long as 150 days. Data were presented in a late-breaking abstract by principal investigator Esther Freeman, MD, PhD, at EADV Virtual on October 29, 2020.1
Analysis from the COVID-19 Dermatology Registry revealed that some patients who develop cutaneous symptoms of the disease become “long haulers” with persistent skin symptoms lasting as long as 150 days. Data were presented in a late-breaking abstract by principal investigator Esther Freeman, MD, PhD, at EADV Virtual on October 29, 2020.1
As of August 2020, 990 cases from 39 countries were recorded, with 303 laboratory-confirmed cases of COVID-19. Of these cases, patients presented with a variety of symptoms, including pernio; vesicular, urticarial, macular erythema, and morbilliform morphologies; and retiform purpura. Previous analysis of the data showed that the rate of hospitalization varied based on COVID-19 skin symptoms; 100% of patients with retiform purpura were hospitalized vs 16% of patients with pernio.2
In the present analysis, all dermatologic symptoms of COVID-19 had an average duration of 12 days, and the laboratory-confirmed subset of cases had an average duration of 7 days.1 Manifestations such as urticaria, morbilliform, and macular erythema lasted for a median of 5 days, 7 days, and 10 days, respectively. More notably, papulosquamous eruptions lasted 20 days, with a few outlier cases lasting as long as 70 days. Pernio/chilblains lasted for a median of 15 days; however, six cases of pernio/chilblains lasted 60 days or more, with one laboratory-confirmed case persisting as long as 150 days.
“I think it’s important to note that the registry data really likely underrepresents the reality, because most of the providers did data entry at one time very shortly after seeing the patient, and if the symptoms were ongoing, the full course may not have been captured,” said Dr Freeman in the presentation. She noted that the research group requested updates from participants twice, but the current results may underrepresent patients with long-lasting symptoms.
The registry reveals further insight into the systemic inflammatory effects of COVID-19 and its persistent effects after recovery from acute infection. Health care providers can continue to submit cases by visiting www.aad.org/covidregistry.
References
1. McMahon DE, Gallman AE, Hruza GJ, et al. COVID-19 "long-haulers" in dermatology? Duration of dermatologic symptoms in an international registry from 39 countries. Late breaking abstract no. 3090. Presented at: EADV Virtual; October 29, 2020; virtual.
2. Freeman EE, McMahon DE, Lipoff JB. The spectrum of covid-19 associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(40):1118-1129. doi:10.1016/j.jaad.2020.06.1016
Patients Report Immediate and Sustained Improvements in Severity, HRQL, and Mental Health in Two Phase 3 Clinical Trials
Data from two replicate phase 3 clinical trials (UltIMMa-1 and UltIMMa-2) revealed that a significant proportion of patients receiving risankizumab achieved improvements in psoriasis severity, quality of life, and mental health measures vs patients who received ustekinumab or placebo.
The study compared the patient-reported outcomes of psoriasis symptoms, health-related quality of life (HRQL), and mental health of patients receiving either risankizumab, ustekinumab, or placebo over 52 weeks in randomized, double-blind, multisite, and placebo- and active comparator-controlled trials.
In total, 997 patients aged 18 years or older who had moderate to severe chronic plaque psoriasis with a body surface area involvement of 10% or more, a Psoriasis Area Severity Index score of 12 or greater, and a statis Physician Global Assessment score of 3 or higher were included. Patients were randomized 3:1:1 to receive either 150 mg of risankizumab or 45 mg or 90 mg of ustekinumab per weight-based dosing instructions for 52 weeks, or matching placebo for 16 weeks followed by risankizumab for the study remainder.
The main outcomes measured were Psoriasis Symptom Scale (PSS), including the total score as well as the item scores for pain, redness, itchiness, and burning; Dermatology Life Quality Index (DLQI); 5-level EuroQoL-5D (EQ-5D-5L); and Hospital Anxiety and Depression Scale (HADS). Outcomes were compared at baseline and again at weeks 16 and 52 of treatment.
At baseline, patient characteristics and reported outcomes were similar across all groups. However, at week 16, a significantly greater proportion of patients treated with risankizumab achieved a PSS score of 0 (no psoriasis symptoms) and DLQI score of 0 or 1 (no impact on skin-related HRQL) compared with the patients treated with ustekinumab or placebo. Further, a significantly greater proportion of the risankizumab group achieved minimally clinically important difference for DLQI, EQ-5D-5L, and HADS (anxiety, depression) at week 16 vs ustekinumab and placebo. At week 52, these improvements were sustained for PSS, DLQI, and EQ-5D-5L.
These results of the UltIMMa-1 and UltIMMa-2 trials on patient-reported outcomes demonstrated the superiority of risankizumab in reducing or even eliminating psoriasis symptoms and psychological distress vs ustekinumab and placebo. The findings, along with previously reported data, demonstrate that risankizumab is an efficacious novel biologic therapy that can lead to clinically meaningful improvements in quality of life in patients with plaque psoriasis.
Reference
Augustin M, Lambert J, Zema C, et al. Effect of risankizumab on patient-reported outcomes in moderate to severe psoriasis. JAMA Dermatol. Published online October 14, 2020. doi:10.1001/jamadermatol.2020.3617



























