Psychosocial Comorbidities in Moderate to Severe AD Associated With QOL Impairment
 A recent study characterized patients with vs without psoriasis in challenging-to-treat areas seen in routine US clinical practices. The results were published in the journal Dermatology.
A recent study characterized patients with vs without psoriasis in challenging-to-treat areas seen in routine US clinical practices. The results were published in the journal Dermatology.
Adult patients with psoriasis who were enrolled in the Corrona Psoriasis Registry between April 2015 and May 2018 and who initiated a biologic therapy were included in the retrospective observational study. Patient demographics, clinical characteristics, disease activity, and patient-reported outcome measures (pain, fatigue, itch, EuroQol visual analog scale [EQ VAS], Dermatology Life Quality Index [DLQI], and Work Productivity and Activity Impairment questionnaire) at registry enrollment were assessed. These characteristics were then compared between patients with vs patients without challenging-to-treat areas. Nonparametric Kruskal-Wallis tests and χ2 or Fisher exact tests were used to analyze continuous variables and categorical variables, respectively. Additionally, the study used generalized linear regression models to estimate differences in disease activity and patient-reported outcomes between patients with vs without each challenging-to-treat area.
Of the 2042 patients with psoriasis included in the analysis, 38.4% had psoriatic arthritis (PsA), 38.1% had scalp psoriasis, 16.0% had nail psoriasis, and 10.9% had palmoplantar psoriasis. Further, 26.2% had a combination of two or more challenging-to-treat areas and PsA, while only 34.2% had body plaque psoriasis without PsA or challenging-to-treat areas. Patients within the challenging-to-treat groups reported higher (mean [95% CI]) itch (scalp, 58.01 [57.62-58.40] vs 54.35 [53.99-54.72]; nail, 56.42 [56.02-56.81] vs 55.59 [55.20-55.97]; palmoplantar, 60.22 [59.86-60.59] vs 55.15 [54.79-55.54]) and lower EQ VAS (scalp, 68.12 [67.78-68.48] vs 69.46 [69.12-69.81]; nail, 66.21 [65.89-66.55] vs 69.48 [69.14-69.83]; palmoplantar, 66.21 [66.07-66.75] vs 69.29 [68.94-69.94]) scores than patients without challenging anatomical locations. In particular, patients with nail or palmoplantar psoriasis reported higher scores for pain, fatigue, and DLQI, and more patients with scalp or palmoplantar psoriasis reported work impairment than those without.
The authors noted that two-thirds of patients with psoriasis who initiated biologic therapy had PsA and/or one or more challenging-to-treat area. Based on the study data, they concluded that “patients with challenging-to-treat areas had worse patient- reported outcome scores than those without, indicating a significant burden of challenging-to-treat areas on patients' quality of life.”
Reference
Callis Duffin K, Mason MA, Gordon K, et al. Characterization of patients with psoriasis in challenging-to-treat body areas in the Corrona Psoriasis Registry. Dermatology. 2021;237(1):46-55. doi:10.1159/000504841
Study Highlights Persistent Need for Generalized Pustular Psoriasis-Specific Treatments
A survey found that there are key features within generalized pustular psoriasis (GPP) flares that could launch discussions around consensus guidelines for diagnosis and management.
GPP is “a rare, severe, and potentially life-threatening systemic and chronic autoinflammatory disease characterized by sterile, neutrophilic pustules.” The standard treatment varies by region, and there is very little information and experience with the flares. To establish current unmet needs in GPP, the study authors analyzed the natural history of GPP, how dermatologists diagnose GPP including GPP flares, and the range and adequacy of GPP treatment options currently prescribed by dermatologists. A 28-question structured survey examined 10 themes, ranging from GPP diagnostic criteria to GPP symptoms and treatment, and was completed by 29 eligible dermatologists.
According to the results of the survey, all respondents indicated that pustules were necessary to diagnose a GPP flare. They reported that the most frequent triggering factors for GPP were steroid withdrawal (64%), infection (58%), and stress (50%). They also indicated that the available treatment options for GPP flares were adequate most (79%) or all(14%) of the time. Despite this, 38% reported that hospitalization due to flares was somewhat common. Additionally, 72% of dermatologists reported that treatments were too slow to control flares, and 66% stated that treatments, at least sometimes, did not adequately prevent new flares.
The study concluded that, while the results suggest moderately effective therapies exist, there remains a need for GPP- specific treatments.
Reference
Strober B, Kotowsky N, Medeiros R, et al. Unmet medical needs in the treatment and management of generalized pustular psoriasis flares: evidence from a survey of Corrona Registry dermatologists. Dermatol Ther (Heidelb). Published online February 27, 2021. doi:10.1007/s13555-021-00493-0
Psychosocial Comorbidities in Moderate to Severe AD Associated With QOL Impairment
 A study published in Advances in Therapy that evaluated adults with moderate to severe atopic dermatitis (AD) in the United States found a significant link between psychosocial comorbidities and health outcomes, including health status, work loss, and health care resource utilization (HRU).
A study published in Advances in Therapy that evaluated adults with moderate to severe atopic dermatitis (AD) in the United States found a significant link between psychosocial comorbidities and health outcomes, including health status, work loss, and health care resource utilization (HRU).
Using data from the 2017 US National Health and Wellness Survey, the study included patients with a physician diagnosis of moderate to severe AD or eczema. Using generalized linear models, the researchers examined the relationship between psychosocial comorbidities, including sleep difficulties, anxiety, and depression (based on self-report and Patient Health Questionnaire-9, respectively), and health outcomes (36-item Short Form Health Survey, version 2; EuroQol five-dimension, five-level; Work Productivity and Activity Impairment questionnaire; and HRU).
Of the respondents with moderate to severe AD (N=1017), 56.6%, 70.7%, and 60.9% reported sleep difficulties, depression, and anxiety, respectively, wrote the study authors. Each comorbidity was significantly associated with reduced scores and increased overall work impairment (P<.05), as well as increased HRU.
The authors noted that patients with moderate to severe AD often reported these psychosocial comorbidities, which have been found to have a significant link with health status, work loss, and HRU.
Reference
Kwatra SG, Gruben D, Fung S, DiBonaventura M. Psychosocial comorbidities and health status among adults with moderate-to-severe atopic dermatitis: a 2017 US National Health and Wellness Survey analysis. Adv Ther. Published online February 8, 2021. doi:10.1007/s12325-021-01630-z
Cross-Sectional Study Finds Gap in Recognizing and Diagnosing Pediatric Hidradenitis Suppurativa
 An increased awareness and recognition of hidradenitis suppurativa (HS) prevalence in children could help better understand the condition. A study published in JAMA Dermatology sought to describe the demographics, clinical features, treatment, associated comorbidities, and outcomes within a large cohort of pediatric patients with HS.
An increased awareness and recognition of hidradenitis suppurativa (HS) prevalence in children could help better understand the condition. A study published in JAMA Dermatology sought to describe the demographics, clinical features, treatment, associated comorbidities, and outcomes within a large cohort of pediatric patients with HS.
The study carried out an international, multicenter, retrospective review of pediatric patients (aged 1-18 years) diagnosed with HS from 10 dermatology clinics across the United States, Canada, Israel, Australia, and Italy between January 1996 to January 2017.
In total, 481 patients diagnosed with HS (386 girls) were included in the study. The mean (SD) age of disease onset was 12.5 (2.9) years vs a mean age of 14.4 (3.5) years at diagnosis. It was also noted that family history of HS was present in 111 of 271 (41%) patients.
At the beginning of the disease, the signs/symptoms reported were cyst/abscess in 229 (48%) patients, pain/tenderness in 118 (25%) patients, and papules/pustules in 117 (24%) patients. During an initial dermatologic assessment, 233 (48%) patients already had evidence of skin scarring. Additionally, Hurley staging was documented in 288 (60%) patients, including 47% at stage 1, 45% at stage 2, and 8% at stage 3, and comorbid conditions were reported in 406 (85%) patients. Of these conditions, obesity (263 [65%]) and acne (118 [29%]) were the most common. Complications occurred in 378 (79%) patients, with scars or contractures (301/378 [80%]) being the most prevalent.
The authors determined that there is a gap in recognizing and diagnosing pediatric patients with HS. In addition, these pediatric patients are likely to present with other comorbidities. Future prospective, observational, and interventional studies should be completed to better understand the clinical course of and optimal treatments for pediatric HS.
Reference
Liy-Wong C, Kim M, Kirkorian AY, et al. Hidradenitis suppurativa in the pediatric population: an international, multicenter, retrospective, cross-sectional study of 481 pediatric patients. JAMA Dermatol. Published online February 24, 2021. doi:10.1001/jamadermatol.2020.5435
Tapinarof Cream Found Durable, Safe, and Remittive in Phase 3 Trial
 An interim analysis of the ongoing clinical trial PSOARING 3 has found tapinarof cream durable and effective for the treatment of plaque psoriasis. Data from this interim analysis will be included in a future New Drug Application to the FDA later in 2021.
An interim analysis of the ongoing clinical trial PSOARING 3 has found tapinarof cream durable and effective for the treatment of plaque psoriasis. Data from this interim analysis will be included in a future New Drug Application to the FDA later in 2021.
PSOARING 3 is a long-term, open-label extension study to evaluate the safety and efficacy of tapinarof cream 1% for the treatment of plaque psoriasis in adults. In the trial, patients who had previously completed treatment with tapinarof or vehicle in either the PSOARING 1 or PSOARING 2 pivotal efficacy and safety studies received tapinarof cream for up to 52 weeks. The preplanned interim analysis was performed once 100 participants received tapinarof for 52 weeks and another 300 received the topical for 26 weeks.
This early analysis found 57.3% of patients who entered the study with a Physician Global Assessment (PGA) score of 2 or more achieved a PGA score of 0 or 1, indicating a therapeutic effect beyond the initial 12-week treatment period. Further, 39.2% achieved com- plete clearance (PGA=0), and there was no evidence of tachyphylaxis.
For patients who entered PSOARING 3 with a PGA score of 0, the median time to disease worsening (defined as PGA ≥2) was approximately 115 days.
As for safety, discontinuation due to adverse events (AEs) at the time of the interim analysis (5.8%) was consistent with the PSOARING 1 and PSOARING 2 pivotal trials (5.6% and 5.8%, respectively). In addition, no increased risk of AEs were observed with the long-term use of tapinarof cream.
Reference
Positive data from PSOARING 3 support long-term use of tapinarof cream in adults with plaque psoriasis, with durable (on-therapy) and remittive (off-therapy) benefits. News release. Dermavant; February 18, 2021. Accessed February 19, 2021. https://www.businesswire.com/news/home/20210218005582/en/Positive-Data-from- PSOARING-3-Support-Long-Term-Use-of-Tapinarof-Cream-in-Adults-with-Plaque- Psoriasis-with-Durable-On-Therapy-and-Remittive-Off-Therapy-Benefits
Phase 3 Trial Finds Tirbanibulin Effective for AKs, More Long-term Data Needed
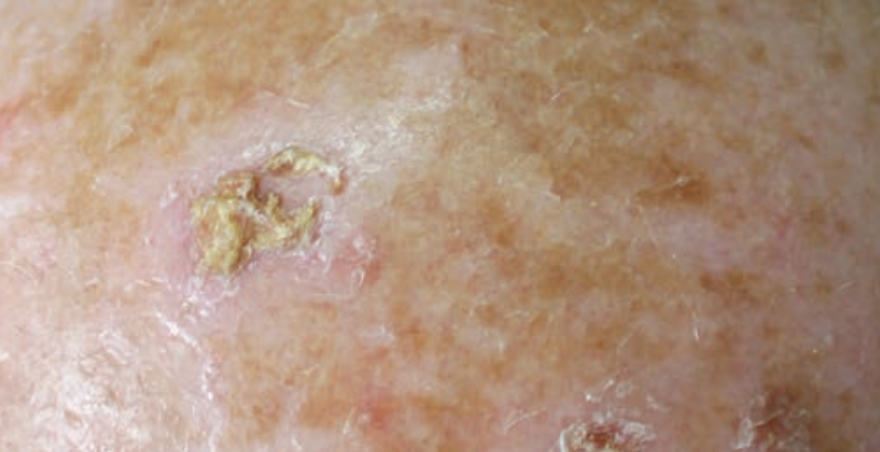 A study published in New England Journal of Medicine found tirbanibulin was superior to vehicle as a topical treatment for actinic keratoses (AKs).
A study published in New England Journal of Medicine found tirbanibulin was superior to vehicle as a topical treatment for actinic keratoses (AKs).
Adults with AKs on the face or scalp were randomly assigned to receive either topical tirbanibulin 1% or vehicle (placebo) ointment in two identically designed double-blind trials at a 1:1 ratio. Once per day for 5 consecutive days, patients applied the ointment to a 25-cm2 contiguous area containing four to eight lesions. After 57 days of treatment, the primary outcome evaluated was the percentage of patients with complete reduction in the number of AKs and the secondary outcome was percent- age of patients with a partial (≥75%) reduction. Local reactions were scored with the use of 4-point scale (ranging from 0 [ab- sent] to 3 [severe]), and after 1 year, incidence of recurrence was evaluated.
Each trial consisted of 351 patients (N=702). In trial 1, 44% (77/175) of the patients in the tirbanibulin group had complete clearance vs only 5% (8/176) in the vehicle group (95% CI, 32-47; P<.001). For trial 2, these percentages increased to 54% (97/178) and 13% (22/173) patients (95% CI, 33-51; P<.001). These percentages revealed that patients with partial clearance were significantly higher in the tirbanibulin groups than in the vehicle groups.
At 1 year, it was estimated that 47% of patients with recurrent lesions previously achieved a complete response to tirbanibulin. Common local reactions to tirbanibulin were erythema (91%) and flaking or scaling (82%); adverse events experienced were application-site pain (10%) and pruritus (9%), all of which resolved.
Based on the results of these two trials, tirbanibulin applied once daily for 5 days was superior to vehicle for the treatment of AKs after 2 months, though the treatment was associated with transient local reactions and recurrence of lesions at 1 year. Further trials to compare tirbanibulin with conventional treatments and with longer follow-up are needed.
Reference
Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. Published online February 11, 2021. doi:10.1056/NEJMoa2024040
























