Palmoplantar Pustulosis Has Significant Impact on Patient QOL vs Plaque Psoriasis
 Palmoplantar pustulosis (PPP) has a significant impact on daily activities, including greater itch, fatigue, and pain, than plaque psoriasis alone, according to a poster presented at the American Academy of Dermatology Virtual Meeting Experience 2021.
Palmoplantar pustulosis (PPP) has a significant impact on daily activities, including greater itch, fatigue, and pain, than plaque psoriasis alone, according to a poster presented at the American Academy of Dermatology Virtual Meeting Experience 2021.
Classification of PPP as psoriasis is debated amongst researchers due to limited information on the natural history of the disease. Thus, therapeutic options for patients with PPP are limited. Treatments currently used to treat PPP were originally developed for other forms of psoriasis and are not designed to specifically target PPP.
Data from the Corona Psoriasis Registry, a prospective, multicenter, noninterventional registry, was used for this descriptive, cross-sectional study. In total, 64 patients with PPP were compared to 4894 patients with plaque psoriasis. Data gathered included sociodemographics, disease characteristics, patient-reported outcomes (PROs), and medication use.
Analyzing demographics, the proportion of patients with comorbidities between patients with PPP and plaque psoriasis was similar. In both populations, 14% had diabetes, hypertension occurred in 41% of patients with PPP and 37% of patients with plaque psoriasis, and anxiety and/or depression occurred in 29% of patients with PPP and 26% of patients with plaque psoriasis.
As expected, due to the nature of localized pathology, patients with PPP had a lower mean body surface area (BSA; 5%) compared with patients with plaque psoriasis (10%). Based on BSA affected, 56.3% of patients with PPP were classified as having moderate/severe disease compared 63.5% of patients with plaque psoriasis. However, when using visual analog scores (VAS) to assess disease severity, patients with PPP had a higher median and mean VAS score vs individuals with plaque psoriasis. Patients with PPP experienced itch, fatigue, and pain more frequently than patients with plaque psoriasis.
Utilizing the Patient Global Assessment as a PRO measure, patients with PPP reported a median impact of 43 vs a
median of 30 in patients with plaque psoriasis. Additionally, the median reported percentage of impairment while working (10 vs 0) and in daily activities (30 vs 3) was greater for patients with PPP than those with plaque psoriasis.
Despite occurring less frequently and affecting a smaller population, a diagnosis of PPP has a great impact on an individual’s life. Patients with PPP experience a larger negative impact on the quality of life. This highlights the need for targeted therapies for PPP.
Reference
Lebwohl M, Kotowsky N, Medeiros RA, et al. Greater disease burden associated with palmoplantar pustulosis versus plaque psoriasis: real-world evidence from the North American-based Corrona Psoriasis Registry. Poster presented at: American Academy of Dermatology Virtual Meeting Experience 2021; April 23-25, 2021; virtual.
SPONSORED CONTENT
 New Emollient Cream Improves TEWL, Skin Viscoelasticity
New Emollient Cream Improves TEWL, Skin Viscoelasticity
A novel skin moisturizing formulation utilizing a natural beeswax base and emollient ingredients was found to improve transepidermal water loss (TEWL) and skin viscoelasticity in atopic skin, following a 4-week, open-label study. No adverse events were recorded following use, demonstrating a safe, well-tolerated product for use by patients with atopic dermatitis.
Over the course of the study, 12 participants with mild to moderate eczema applied the skin moisturizing formulation twice daily for 4 weeks. TEWL and corneometry were assessed at a target site during the baseline visit and again at 48 hours and 4 weeks. The primary end points were the change in TEWL and cutometer measurements after 4 weeks of moisturizer use.
At baseline, cutometry was 32.1 units for all patients. The measurement improved to 39.3 units (P<.001) at 48 hours and 44.4 units (P<.001) at 4 weeks, increases of 23% and 38%, respectively. Similarly, improvements were seen in TEWL; baseline TEWL was 7.5 g/m2/hr, which decreased by 23% at 48 hours and 22% at 4 weeks (5.8g/m2/hr and 5.8g/m2/hr, respectively).
This novel moisturizing product differs from typical oil-in-water products by using water-in-oil emulsion design with a natural beeswax base to provide an occlusive emollient barrier in a cream formulation. It contains water, mineral oil, microcrystalline wax, acetylated lanolin, beeswax, sorbitan sesquioleate, isopropyl myristate, sodium borate, allantoin, and imidurea.
Reference
Draelos ZD. Skin barrier-restoring effects of an emollient dry skin cream. Article in press.
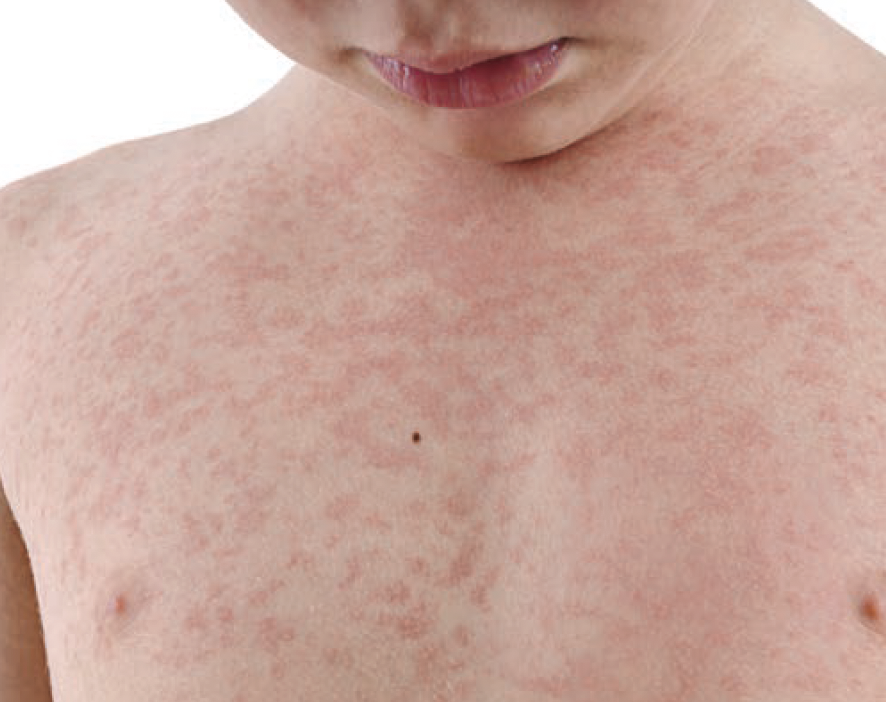 Pediatric Urticaria Resolution Associated With Elevated CD63 Levels and Thyroid Comorbidity
Pediatric Urticaria Resolution Associated With Elevated CD63 Levels and Thyroid Comorbidity
Elevated CD63 levels and thyroid disease are associated with chronic inducible urticaria (CIndU) resolution in pediatric patients, according to a study published in International Archives of Allergy and Immunology.
“We aimed to assess the demographics, clinical characteristics, comorbidities, natural history, and management of pediatric patients with CIndU,” explained the study authors.
Over a 6-year period, children presenting to an allergy clinic with CIndU were analyzed. Researchers defined resolution as the absence of hives for 1 year without treatment.
Of the pediatric cohort, the median age was 12.5 years and 60.3% had cold chronic urticaria and 41.3% had cholinergic chronic urticaria. Second-generation antihistamines controlled 71.4% of the cases. Within the 6-year course of the study, the resolution rate was 45.3% and more likely in patients who presented with well-controlled urticaria control test scores, elevated CD63 counts, and in those with a thyroid comorbidity.
“The natural history of CIndU resolution in pediatric patients was relatively low and was associated with elevated CD63 levels, as well as thyroid comorbidity,” concluded the study authors.
Reference
Miles LM, Gabrielli S, Le M, et al. Clinical characteristics, management, and natural history of chronic inducible urticaria in a pediatric cohort. Int Arch Allergy Immunol. Published online April 1, 2021. doi:10.1159/000514757
Mobile Apps Improve Patient-Provider Interactions When Integrated Into Care Processes
Patient-provider outcomes are more favorable when data from mobile applications measuring patient-reported outcomes are integrated into care processes.
Researchers aimed to explore the effects of apps measuring patient-reported outcomes on patient-provider interaction in the rheumatic diseases.
Patients with either rheumatoid arthritis, axial spondyloarthritis, or psoriatic arthritis were offered mobile apps to track their disease status between rheumatology visits. Researchers compared outcomes among patients who used an app and discussed the app data with their physician, used an app but did not discuss the data with their physician, and did not use an app. Shared decision-making and physician awareness of disease fluctuations were also assessed.
The app plus discussion group rated their rheumatologist higher in shared decision-making (odds ratio [OR] 1.7; 95% CI, 1.1-2.4) and physician awareness of disease fluctuations (OR 2.0; 95% CI, 1.3-3.1) compared with non-app users.
“App users who discussed app data with their rheumatologist reported more [favorably] on patient-provider interactions than app users who did not and non-app users. Apps measuring PROs may contribute little to patient-provider interactions without integration of app data into care processes,” concluded the study authors.
Reference
Shaw Y, Courvoisier DS, Scherer A, et al. Impact of assessing patient-reported outcomes with mobile apps on patient-provider interaction. RMD Open. 2021;7(1):e001566. doi:10.1136/rmdopen-2021-001566
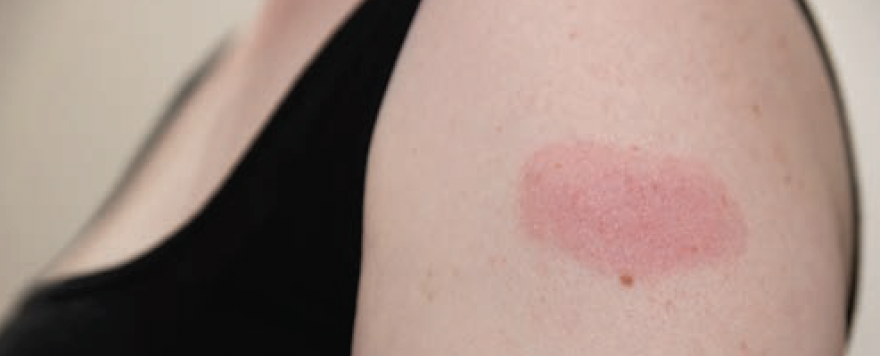 COVID-19 mRNA Vaccines May Cause Minor Cutaneous Reactions
COVID-19 mRNA Vaccines May Cause Minor Cutaneous Reactions
According to evidence from an international registry regarding dermatologic findings related to COVID-19, mRNA vaccines can cause minor cutaneous reactions following injection. In particular, these findings were more commonly reported with the mRNA-1273 (“Moderna”) vaccine than the BNT162b2 (“Pfizer”) vaccine, per the results of the study published in Journal of the American Academy of Dermatology.
“Given the importance of widespread vaccination in curbing the pandemic, we aimed to collect cases of cutaneous side effects to the [mRNA] COVID-19 vaccines (1) to describe the morphology and timing of cutaneous reactions to the Pfizer and Moderna vaccines and (2) to understand differences in cutaneous reactions between the [two] vaccine doses to guide vaccine counseling,” wrote the study authors.
An international registry of cutaneous manifestations of SARS-CoV-2 was established between the American Academy of Dermatology and the International League of Dermatological Societies in March 2020. This registry was expanded in December 2020 to obtain data on cutaneous reactions developing due to the vaccines.
From December 24, 2020, to February 14, 2021, 414 patients reported cutaneous reactions with the Moderna (83%) and Pfizer vaccine (17%). Of these, 21% reported reactions after the first dose, 63% after the second dose, and 16% after both doses. Delayed large local reactions were most common, followed by local injection site reactions, urticarial eruptions, and morbilliform eruptions. Reactions were minor and self-limited.
“We report a spectrum of cutaneous reactions after mRNA COVID-19 vaccines,” wrote the study authors. “We observed some dermatologic reactions to Moderna and Pfizer vaccines that mimicked SARS-CoV-2 infection itself, such as pernio/chilblains,” they added.
Reference
McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. Published online April 7, 2021. doi:10.1016/j.jaad.2021.03.092



























