Diagnosis: Verruciform xanthoma
Verruciform xanthoma (VX) is a benign, wart-like lesion that typically occurs on the oral mucosa. It was given its name in 1971 by oral pathologist William G. Shafer, DDS, MS, who described 15 cases of VX in the oral cavity.1 Extraoral VX has also been described in the anogenital region, appearing on the anus, vulva, penis, and scrotum. Other locations include the esophageal mucosa, ear, nose, extremities, and sacrum.2,3 Oral and extraoral VXs are commonly solitary lesions, but disseminated cases have been described.4 VX has no certain disease associations and is usually asymptomatic.
Epidemiology
VX is a rare lesion mainly described in adults. There have been isolated cases and some cases associated with congenital hemidysplasia with ichthyosiform erythroderma and limb defects (CHILD) syndrome described in the pediatric population; however, these are even more rare.5-7 A literature survey by Philipsen et al8 of 282 oral VX cases from 1979 to 2003 showed the mean age of occurrence to be 54.9 years in females and 44.2 years in males, with an overall female to male ratio of 1:1.1. On closer analysis, a slight male preponderance was seen in patients younger than 50 years and a slight female preponderance seen in patients older than 50 years.8 The cases were mainly Caucasian and Japanese patients, though VX has been described in African Americans, South Americans, and Asians.2,8
Clinical Presentation
Oral and extraoral VXs usually present as asymptomatic, solitary, slow-growing, well-circumscribed lesions (Figure 1). Oral VX appears as yellow, red, or gray plaques that can be raised or depressed. Cutaneous VX usually occurs in the anogenital region and appears as pink or gray plaques or pedunculated nodules.8 The surface of both oral and extraoral VX can appear “pebbly” or “mulberry-like.”9,10 Isolated VX generally ranges from 0.2 to 2 cm in diameter, but larger lesions in patients with CHILD syndrome and dystrophic epidermolysis bullosa (DEB) have been described.7

Dermoscopic imaging of VX has revealed two characteristic features. First, each papilla contains hairpin vessels, demonstrating dilated vessels in the stroma. The hairpin vessels are surrounded by a peripheral white border, which signifies an acanthotic epidermis. The second feature is seen by dermoscopic compression, revealing yellow dots and debris. This finding indicates the presence of lipid-laden foam cells.10-12 Additionally, the use of reflectance confocal microscopy (RCM) in VX has been described by Arzberger et al.11 A sharp demarcation between VX and the surrounding skin with the VX surface showing a loss of honeycomb pattern is seen. Dilated vessels with homogenous material corresponding to the presence of foam cells are seen in the dermis.11 In addition to history and clinical examination, dermoscopy and RCM can help focus the differential diagnosis.
Contrary to other xanthoma subtypes, VX is not typically associated with dyslipidemia and is asymptomatic at presentation. Disease associations have been described and are outlined in Table 1,6,7,9,13-17 though VX is not pathognomonic for any single diagnosis.7
Pathogenesis
The clinical appearance and location of VX can appear similar to condylomatous disease caused by human papillomavirus (HPV). However, HPV and VX do not have a consistent association.18 It is now proposed that VX is a mucocutaneous reaction to local trauma occurring in tandem with a chronic inflammatory process, including graft-vs-host disease, discoid lupus erythematosus, pemphigus vulgaris, lichen planus, chronic lymphedema, and DEB.9,13,14 Multiple VXs appearing after bone marrow transplant have also been described.19 Although the etiology and pathogenesis of VX are poorly understood, the histopathologic appearance is definitive in guiding diagnosis.

Pathology
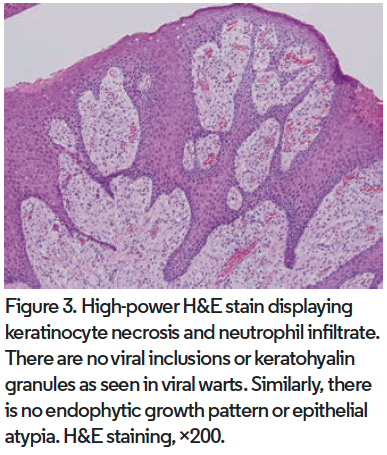
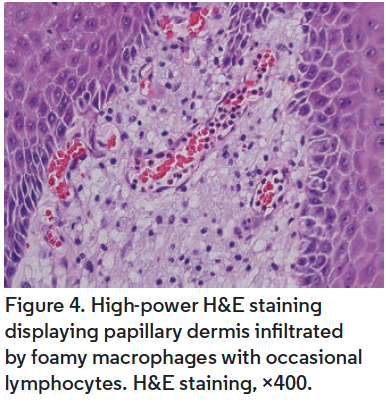
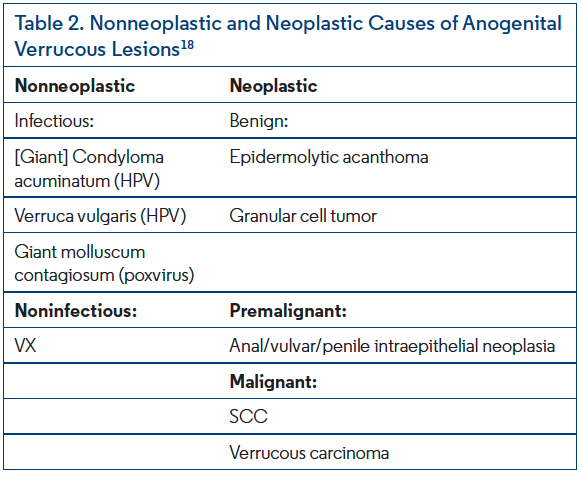
Distinctive features at low magnification of VX include papillomatous acanthosis of the epidermis with parakeratosis and hyperkeratosis (Figure 2). Closer examination reveals keratinocyte necrosis and intracorneal neutrophilic infiltrate (Figure 3). Vacuolated (foamy) macrophages are found infiltrating the papillary dermis with occasional lymphocytes and are hallmark findings in VX (Figure 4). Vascular ectasia is often seen below VX in the dermis as well (Figure 2). VX can be distinguished from infectious and malignant lesions through histopathological examination. There are no viral inclusions or keratohyalin granules as seen in viral warts. Furthermore, there is no endophytic growth pattern or epithelial atypia that would be seen in verrucous carcinoma (Figure 3). Additionally, immunohistochemical analysis has been studied for use in diagnosis of oral VX. CD68, CD63, and CD163 positivity and S100 negativity is found in the classic foam cells of VX and can help in diagnosis. VX is also periodic acid-Schiff positive.2,3,5,18,20
The above features of VX have been organized into three subtypes. Type A (verrucous) shows a predominantly warty pattern, type B (papillary) shows finger-like projections, and type C (flat) shows flat and uniform depth. Often, features of multiple subtypes can be seen in a single lesion, which can be described as a mixed pattern. In a single case report describing VX of the breast, a cystic pattern was noted.21 However, it was concluded that this pattern may have arisen due to its location and not as a reflection of an additional VX structure.21-23
Differential Diagnosis
Determining the diagnosis of a single or multiple anogenital lesions begins with a thorough history and physical examination. The differential diagnosis for anogenital VX can be divided into neoplastic and nonneoplastic causes and is outlined in Table 2.18 Because VX is rare and can often appear clinically similar to more concerning lesions of the anogenital region, a biopsy is necessary to confirm the diagnosis. Patients with varying HPV strains are predisposed to develop premalignant and malignant lesions. Low-risk HPV type 6 and 11 are commonly responsible for the development of condyloma acuminatum, whereas high-risk HPV type 2 and 16 can cause verruca vulgaris and SCC, respectively. HPV genotyping is therefore an important study where diagnosis is equivocal. The most important distinguishing features of VX clinically are location and gross appearance; however, on histopathologic analysis the presence of vacuolated (foamy) macrophages infiltrating the papillary dermis and the lack of epithelial atypia stand to confirm the diagnosis.2,18
Despite the above differentiating factors, VX and SCC can occur simultaneously as described by Takiwaki et al.15 A misdiagnosed penile VX was treated as such and developed into overt SCC 6 years later. On closer retrospective histopathologic analysis, features of SCC were seen arising within the initial VX lesion. This kind occurrence shows the importance of careful microscopic examination. It also shows the need for follow-up.15
Treatment and Prognosis
VX are generally slow-growing, asymptomatic lesions and, if left untreated, can be present for many months to years before intervention is sought.2 Surgical excision is curative with low rates of recurrence. A small number of case reports have described the successful use of adjuvant therapy following surgery in the form of topical imiquimod and fractionated carbon dioxide laser treatment.5,20 VX are not locally aggressive or invasive and prognosis is very good.
Our Patient
Shave biopsy of this patient’s scrotal mass was sent for histopathologic analysis. The results were classic for and confirmed VX. Ten days following the biopsy, the patient was contacted and reported doing well without pain, bleeding, or discharge.
Conclusion
VX is a rare lesion found in oral and extraoral locations. While it is benign, VX can mimic the appearance of more serious masses and must undergo surgical excision to confirm diagnosis and for definitive treatment. Most often this approach is curative without recurrence and avoids misdiagnosis.
Dr Paka is an internal medicine intern at Mount Sinai St. Luke’s & Mount Sinai West in New York, NY. Dr Alapati is chief of dermatology and a dermatopathologist at Brooklyn Veterans Hospital in Brooklyn, NY. Dr Kazlouskaya is a dermatologist and dermatopathology fellow at SUNY Downstate Medical Center, department of dermatology, in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships.
References
1. Shafer WG. Verruciform xanthoma. Oral Surg Oral Med Oral Pathol. 1971;31(6):784-789. doi:10.1016/0030-4220(71)90134-4
2. Stiff KM, Cohen PR. Vegas (verruciform genital-associated) xanthoma: a comprehensive literature review. Dermatol Ther (Heidelb). 2017;7(1):65-79. doi:10.1007/s13555-016-0155-0
3. Gill BJ, Chan AJ, Hsu S. Verruciform xanthoma. Dermatol Online J. 2014;20(1):21253.
4. Tang R, Kopp SA, Cobb C, Halpern AV. Disseminated verruciform xanthoma: a case report. Cutis. 2014;93(6):307-310.
5. Guo Y, Dang Y, Toyohara JP, Geng S. Successful treatment of verruciform xanthoma with imiquimod. J Am Acad Dermatol. 2013;69(4):e184-e186. doi:10.1016/j.jaad.2013.04.026
6. Fedda F, Khattab R, Ibrahim A, Hayek S, Khalifeh I. Verruciform xanthoma: a special epidermal nevus. Cutis. 2011;88(6):269-272.
7. Kurban M, Abbas O, Ghosn S, Kibbi AG. Late evolution of giant verruciform xanthoma in the setting of CHILD syndrome. Pediatr Dermatol. 2010;27(5):551-553. doi:10.1111/j.1525-1470.2010.01276.x
8. Philipsen HP, Reichart PA, Takata T, Ogawa I. Verruciform xanthoma--biological profile of 282 oral lesions based on a literature survey with nine new cases from Japan. Oral Oncol. 2003;39(4):325-336. doi:10.1016/s1368-8375(02)00088-x
9. Cumberland L, Dana A, Resh B, Fitzpatrick J, Goldenberg G. Verruciform xanthoma in the setting of cutaneous trauma and chronic inflammation: report of a patient and a brief review of the literature. J Cutan Pathol. 2010;37(8):895-900. doi:10.1111/j.1600-0560.2009.01470.x
10. Ogata D, Tsuchida T. Characteristic dermoscopic features of verruciform xanthoma: report of three cases. J Dermatol. 2015;42(11):1103-1104. doi:10.1111/1346-8138.13034
11. Arzberger E, Oliveira A, Hofmann-Wellenhof R, Zalaudek I, Cerroni L, Komericki P. Dermoscopy and reflectance confocal microscopy in verruciform xanthoma of the glans penis. J Am Acad Dermatol. 2015;72(6):e147-e149. doi:10.1016/j.jaad.2015.02.1094
12. Ohnishi T, Shiraishi H, Fukaya S, Tanaka T, Watanabe S. Verruciform xanthoma: report of three patients with comparative dermoscopic study. Clin Exp Dermatol. 2015;40(2):156-159. doi:10.1111/ced.12503
13. Theofilou VI, Sklavounou A, Argyris PP, Chrysomali E. Oral verruciform xanthoma within lichen planus: a case report and literature review. Case Rep Dent. 2018;2018:1615086. doi:10.1155/2018/1615086
14. Roy SF, Prokopetz R, Ayroud Y, Pickett L, Litvinov IV. Wart on fire: a rare entity of verruciform xanthoma arising on a lower leg in a setting of chronic lymphedema. JAAD Case Rep. 2017;3(1):36-38. doi:10.1016/j.jdcr.2016.12.002
15. Takiwaki H, Yokota M, Ahsan K, Yokota K, Kurokawa Y, Ogawa I. Squamous cell carcinoma associated with verruciform xanthoma of the penis. Am J Dermatopathol. 1996;18(5):551-554. doi:10.1097/00000372-199610000-00017
16. Wu JJ, Wagner AM. Verruciform xanthoma in association with milroy disease and leaky capillary syndrome. Pediatr Dermatol. 2003;20(1):44-47. doi:10.1046/j.1525-1470.2003.03010.x
17. Fite C, Plantier F, Dupin N, Avril MF, Moyal-Barracco M. Vulvar verruciform xanthoma: ten cases associated with lichen sclerosus, lichen planus, or other conditions. Arch Dermatol. 2011;147(9):1087-1092. doi:10.1001/archdermatol.2011.113
18. Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143(7):821-831. doi:10.5858/arpa.2018-0039-RA
19. Xue R, Su W, Pei X, Huang L, Elbendary A, Chen Z. Multiple verruciform xanthomas following bone marrow transplant. Indian J Dermatol Venereol Leprol. 2016;82(2):208-209. doi:10.4103/0378-6323.164214
20. Joo J, Fung MA, Jagdeo J. Successful treatment of scrotal verruciform xanthoma with shave debulking and fractionated carbon dioxide laser therapy. Dermatol Surg. 2014;40(2):214-217. doi:10.1111/dsu.12382
21. Lomas-García J, Miguélez-Simón A, Cuesta A, Izquierdo FM. Cystic verruciform xanthoma of the breast: an undescribed location of an uncommon lesion. Am J Dermatopathol. 2014;36(3):276-278. doi:10.1097/DAD.0b013e3182957bae
22. Kimura M, Ohto H, Shibata A, Enomoto A, Umemura M. Clinicopathological and immunohistochemical characteristics of verruciform xanthoma of the lower gingiva: a case report. J Clin Diagn Res. 2016;10(6):ZD05-ZD06. doi:10.7860/JCDR/2016/15446.7950
23. Nowparast B, Howell FV, Rick GM. Verruciform xanthoma. A clinicopathologic review and report of fifty-four cases. Oral Surg Oral Med Oral Pathol. 1981;51(6):619-625. doi:10.1016/s0030-4220(81)80012-6































