Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease with constantly evolving therapies and management. Prevalence of AD is continuously increasing, with 15% to 20% of children and 1% to 3% of adults currently affected worldwide.1 Although it can occur at any age, AD most commonly develops between the ages of 3 and 6 months. This condition not only affects a patient’s physical appearance, but it also imposes a considerable burden on patient physical, mental, and overall wellbeing, and it can be a burden for caregivers as well.1,2 As with any disorder, the ideal treatment regimen for AD is one that takes into consideration a patient’s underlying risk factors and potential barriers to treatment when considering feasible therapies. Treatment of this disease becomes more complex when considering the substantial burden of AD and the underlying controversies surrounding AD risk factors, available therapies, and reasons for treatment failure. This article aims to shed light on the complexities involved in the management of AD and provide avenues for potential new research.
Risk Factors
Controlling for risk factors and minimizing exposure to irritants are important in the treatment of AD. The relationship between AD and factors such as skin exposure to hard and soft water, breastfeeding, and the effect of probiotics on the composition of the gut microbiota is complex and often complicated by conflicting data (Table3-26).
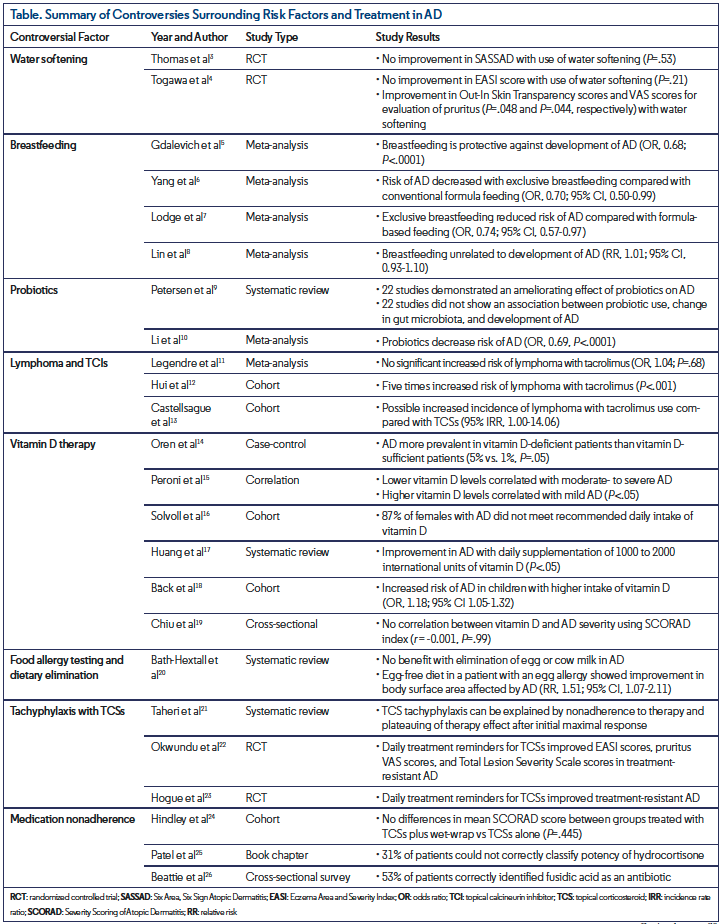
According to some observational studies, the use of hard water is associated with the development of AD.27,28 Hard water contains an appreciable amount of dissolved minerals, such as calcium and magnesium, while soft water contains minimal levels.27 Geographic areas where the use of hard water is more common have increased prevalence and more frequent exacerbations of AD.28 Hard water is postulated to impair the skin’s natural barrier, resulting in increased exposure to minerals as well as sodium lauryl sulfate (SLS), an anionic detergent and surfactant found in many personal hygiene products, such as toothpaste and soaps, and a well-known irritant of AD.27 Additionally, an increase in SLS deposition after the use of hard water positively correlates with an increase in transepidermal water loss, a predictive biomarker for AD (P<.0001).27 Water softening through ion exchange removes the calcium and magnesium ions in hard water and reduces irritation by SLS,27 although the use of water-softening devices in the prevention and management of AD remains controversial.3,29 In a 2011 controlled trial, 334 participants were randomized into two groups for 12 weeks of treatment with either a water-softening device in addition to standard treatment for AD or standard AD treatment alone.3 The group that received a water-softening device experienced a mean five-point drop in their SASSAD severity score, a severity index intended to assess response to treatment in therapeutic trials and to monitor disease activity in AD. The standard care group experienced a mean 5.7-point drop in their SASSAD score, with a negligible difference of 0.66 among the two groups in favor of the standard of care treatment group (P=.53).3 In a small randomized controlled pilot study of 12 patients with childhood AD, the use of soft water treatment compared with placebo did not improve EASI scores (P=.21) or transepidermal water loss from the abdomen and upper back (P=.14 and P=.79, respectively).4 However, there was improvement in the means of Out-In Skin Transparency scores, a measurement of permeability and penetration due to skin dysfunction in the stratum corneum (P=.048), and the VAS scores for evaluation of pruritus (P=.044).4,30 Thus, this study recommended the use of water softening in the treatment of mild to moderate AD despite the lack of improvement in disease severity.4 Although water softening may decrease the amount of minerals in water as well as minimize the SLS deposition, there is insufficient evidence to support the use of such devices as an effective treatment for AD,2 as only one study to date researching this topic has managed to recruit more than 12 participants.3 Larger intervention studies may help to determine the efficacy of water softening as a viable preventative measure for AD.
Breastfeeding is another variable postulated to affect the development of AD. Although breastfeeding is universally recommended for new mothers, there are conflicting data on the relationship between breastfeeding and AD. According to a meta-analysis of 18 prospective studies, breastfeeding was protective against the development of AD (OR, 0.68; P<.0001),5 but in a more recent meta-analysis comparing exclusive breastfeeding with conventional formula feeding, there was a decreased risk of AD in the breastfed group (OR, 0.70; 95% CI, 0.50-0.99).6 In an alternate study, exclusive breastfeeding again reduced the risk of AD when compared with formula-based feeding (OR, 0.74; 95% CI, 0.57-0.97).7 However, in a 2019 meta-analysis, breastfeeding and the future development of AD were unrelated (RR, 1.01; 95% CI, 0.93-1.10).8 Breastfeeding has many immunomodulatory factors that strengthen an infant’s immune system, protecting against infection. As infection can precede the development of atopy, this protection may explain the potentially protective effect of breastfeeding in AD.6
Probiotics may play a potential preventative role in the development of AD; however, there is concern regarding the efficacy of certain strains and the extent to which gut microbiota plays a role in AD. While current literature has established an association between the gut microbiota and other immune-related diseases, there is inconclusive evidence to support such a relationship between the gut microbiota and AD.9 Out of 44 studies in a 2018 systematic review, five reported no difference in the gut microbiota of healthy participants vs patients with AD (P<.05), four studies reported a less diverse microbiota in patients with AD (P<.05), and one study reported an increase in the risk of AD with increased diversity (P<.05).9 This systematic review also evaluated the effects of probiotics on AD, with approximately half of the studies demonstrating an ameliorating effect of probiotics on AD and a change in gut microbiota composition with a higher concentration of probiotic strains. However, the remaining half of the studies did not show an association between probiotic use, change in gut microbiota, and development or severity of AD. The various studies of different populations in this systematic review present contradictory evidence on the role of the gut microbiota and probiotics in AD. In a more targeted 2019 meta-analysis of 28 studies focused on healthy infants and children, probiotics decreased the risk of AD (OR, 0.69; P<.0001).10 Ultimately, the use of probiotics for the prevention and treatment of AD remains contentious since the efficacy and side effects are not yet well-defined.
Therapies
Various factors, such as evolving therapeutic recommendations, supplemental treatments, patient preferences and ability to adhere to treatment regimens, and potential adverse events of therapies, should be considered when treating AD. More specifically, there is controversy surrounding the side effects of TCIs and the roles of dietary elimination, vitamin D, and food allergy testing in AD.
Currently, TCSs are first-line treatment and TCIs are second-line treatment for AD; these therapies may be associated with an increased risk of malignancy. In 2006, the FDA issued a black-box warning for malignancy, including lymphoma, with the use of the TCIs tacrolimus and pimecrolimus.31 However, according to a 2015 meta-analysis, there was no significant increased risk of lymphoma with exposure to tacrolimus (OR, 1.04; P=.68).11 In contrast, a 2009 study reported a five-times increased risk of lymphoma with the use of tacrolimus (P<.001).12 This study, however, may have involved a misclassification bias as the lymphoma diagnosed while using tacrolimus was cutaneous T-cell lymphoma, a common differential diagnosis of AD.11,12 More recent comparisons of tacrolimus vs TCS use in children with AD suggest a possible increased incidence of lymphoma with tacrolimus compared with TCSs (95% CI corrected IRR, 1.00-14.06).13 Limitations to this cohort study included not accounting for the increased risk of lymphoma with increased severity of AD, resulting in a possible confounding factor, as patients with increased severity are more frequently treated with TCIs.13 TCIs remain a fundamental treatment option for AD as they exhibit minimal systemic absorption and adverse events as well as greater long-term improvement of disease flares compared with TCSs.31
Supplemental vitamin D is postulated to play a role in the epidermal differentiation and natural barrier function of the skin.32 In a study of 290 obese patients, vitamin D-deficient patients reported AD more often than vitamin D-sufficient patients (5% vs 1%; P=.05).14 According to a 2011 study, lower vitamin D levels correlated with moderate to severe AD and higher vitamin D levels correlated with mild AD (P<.05).15 In an earlier study detailing the dietary habits of patients with AD, 87% of women did not meet the daily recommended vitamin D intake of 5 µg.16 In a systematic review, two 4-week randomized controlled trials and two 12-week cohort studies demonstrated improvement in AD with daily supplementation of 1000 to 2000 international units of vitamin D (P<.05).17 However, some studies show the opposite or no association at all. One study reported an increased risk of AD in children with a higher intake of vitamin D after adjusting for family history of atopic diseases (OR, 1.18; 95% CI, 1.05-1.32).18,33 In a 2013 study of 94 children, vitamin D status did not correlate with AD severity, using the SCORAD index (r = -0.001; P=.99).19 These studies did not account for potential confounders such as age, season, geographic area, and dose and duration of vitamin D.17 Although data are conflicting, supplementation of vitamin D in AD may aid in therapy and should be evaluated on an individual basis.
Individuals with a history of atopy often experience an atopic march, a term that describes the sequential development of allergic diseases such as AD, food allergies, allergic rhinitis, and asthma.2 Food allergies based on individual skin-prick tests can trigger AD and, conversely, AD can sensitize a patient to food allergies; although, this relationship is not as pronounced in adults.34-36 As a result, food allergy testing and dietary elimination are commonly utilized in the treatment of AD. In a 2006 study, 17 children with known food allergies underwent an oral food challenge upon resolution of prior AD eruptions, resulting in a new AD eruption in only one child.37 Thus, although food allergies may be linked with AD, this association is generally infrequent, thus the benefit of dietary elimination is questionable. Patients perceive food allergy, unhealthy diet, and AD to be interrelated entities, which may result in the elimination of certain foods without the proper guidance of a physician.36 Studies illustrating a positive association of fast food with AD may drive some of these sentiments,38 although adiposity may play a confounding role.28 In a 2008 systematic review, nine randomized controlled trials with a total of 421 participants demonstrated no benefit with the elimination of egg or cow milk in participants.20 However, an egg-free diet in a patient with an egg allergy showed improvement in body surface area affected by AD (RR, 1.51; 95% CI, 1.07-2.11).20 Elimination diets, though seemingly harmless, have potential risks. Slowed growth due to malnutrition and the development of IgE-mediated allergy are two complications that can result from dietary elimination.36 Thus, understanding the benefits of additional testing may improve AD treatment outcomes while reducing the physical burden on patients.
TCSs remain a mainstay treatment of AD. Although an effective medication, TCSs are susceptible to tachyphylaxis, the gradual reduction in efficacy after successive doses of a medication, which may result in a premature switch to an alternate medication. However, there is conflicting evidence on the development of tachyphylaxis. Even though downregulation of corticosteroid receptors in the skin can occur upon prolonged treatment with TCSs, this may not be the reason for reduced efficacy in all patients.21 In a 2013 systematic review, nonadherence to treatment and the plateauing of therapeutic effect after achieving initial maximal response were two suggested reasons for the development of resistance to TCSs and relapse of disease.21,39 Two studies containing 12 participants each demonstrated this theory of nonadherence. Patients with AD who previously failed TCS treatment experienced improvement in EASI scores, pruritus VAS scores, and Total Lesion Severity Scale scores with desoximetasone 0.25% spray and daily phone-call reminders.22,23 These studies concluded that the apparent decreased efficacy of TCSs was more likely due to poor treatment adherence than to decreased response to medication. Potential issues related to adherence should be considered before discontinuing a treatment and attributing a lack of efficacy to tachyphylaxis.
Failure of appropriate treatment response may also be due to the complexity of a patient’s treatment regimen, as suggested by disease guidelines, rather than the medication itself. For example, the application of wet-wraps, a time-
consuming and often frustrating process, is an effective treatment utilized in cases resistant to topical therapies.40 Yet, a study investigating 50 children with AD demonstrated no difference in mean SCORAD scores between groups treated with TCSs plus wet-wraps or with TCSs alone (P=.445).24 Wet-wrap therapy is a difficult procedure in which individuals may struggle in accurately applying the treatment, suggesting nonadherence as a possible explanation for such findings.25,41 Additionally, when treating worsening AD, physicians often follow treatment guidelines through a practical approach via common guidelines such as the Atopic Dermatitis Yardstick.42 However, this approach assumes proper use of treatments as well as providing a full understanding to patients of the medications involved, their potency, purpose, and appropriate dosing. With such variable components, it can be difficult for patients to keep track of their treatment regimens. It may be more beneficial and effective to consider simpler treatment regimens as a means to improve patient adherence and achieve desired outcomes. When evaluating novel as well as older therapies for AD, the complexity of the management strategy as well as the patient’s preferences and ability to adhere to treatment should be taken into consideration.
Conclusion
Conflicting studies may result in differing recommendations by providers, potentially increasing physical and mental burdens to the AD patient population. Controversial therapies have the potential to bring harm to patients, and this becomes increasingly complicated in instances where conflicting data may present the same therapy as both a protective and risk factor for a disease. Warnings, controversies, complex treatment strategies, and conflicting evidence may affect a patient’s ability to follow recommendations and
appropriately use prescribed medications.
Although this review does not cover all controversies associated with treatment of AD, it highlights several important topics that may be of concern to both patients with AD and providers. Future research aimed at dispelling these controversies has the potential to aid in the effective and safe management of AD. n
Mr Pandher is a medical student and student researcher at the Center for Dermatology Research in the department of dermatology at Wake Forest School of Medicine in Winston-Salem, NC. Ms Ghamrawi is a medical student and research fellow at the Center for Dermatology Research in the department of dermatology at Wake Forest School of Medicine. Ms Heron is a medical student and research fellow at the Center for Dermatology Research in the department of dermatology at Wake Forest School of Medicine. Dr Feldman is with the Center for Dermatology Research and the departments of dermatology, pathology, and social sciences & health policy at Wake Forest University School of Medicine and the department of dermatology at the University of Southern Denmark in Odense, Denmark.
Disclosure: Dr Feldman has received research, speaking and/or consulting support from a variety of companies including Galderma, GSK/Stiefel, Almirall, Leo Pharma, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He is founder and majority owner of www.DrScore.com and founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. Mr Pandher, Ms Ghamrawi, and Ms Heron have no relevant financial relationships.
References
1. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16. doi:10.1159/000370220
2. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218-1233. doi:10.1016/j.jaad.2014.08.038
3. Thomas KS, Koller K, Dean T, et al. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: the Softened Water Eczema Trial (SWET). Health Technol Assess. 2011;15(8):v-vi, 1-156. doi:10.3310/hta15080
4. Togawa Y, Kambe N, Shimojo N, et al. Ultra-pure soft water improves skin barrier function in children with atopic dermatitis: a randomized, double-blind, placebo-controlled, crossover pilot study. J Dermatol Sci. 2014;76(3):269-271. doi:10.1016/j.jdermsci.2014.10.009
5. Gdalevich M, Mimouni D, David M, Mimouni M. Breast-feeding and the onset of atopic dermatitis in childhood: a systematic review and meta-analysis of prospective studies. J Am Acad Dermatol. 2001;45(4):520-527. doi:10.1067/mjd.2001.114741
6. Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol. 2009;161(2):373-383. doi:10.1111/j.1365-2133.2009.09049.x
7. Lodge CJ, Tan DJ, Lau MXZ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):38-53. doi:10.1111/apa.13132
8. Lin B, Dai R, Lu L, Fan X, Yu Y. Breastfeeding and atopic dermatitis risk: a systematic review and meta-analysis of prospective cohort studies. Dermatology. 2020;236(4):345-360. doi:10.1159/000503781
9. Petersen EBM, Skov L, Thyssen JP, Jensen P. Role of the gut microbiota in atopic dermatitis: a systematic review. Acta Derm Venereol. 2019;99(1):5-11. doi:10.2340/00015555-3008
10. Li L, Han Z, Niu X, et al. Probiotic supplementation for prevention of atopic dermatitis in infants and children: a systematic review and meta-analysis. Am J Clin Dermatol. 2019;20(3):367-377. doi:10.1007/s40257-018-0404-3
11. Legendre L, Barnetche T, Mazereeuw-Hautier J, Meyer N, Murrell D, Paul C. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72(6):992-1002. doi:10.1016/j.jaad.2015.02.1116
12. Hui RL, Lide W, Chan J, Schottinger J, Yoshinaga M, Millares M. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43(12):1956-1963. doi:10.1345/aph.1M278
13. Castellsague J, Kuiper JG, Pottegård A, et al. A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation - JOELLE study). Clin Epidemiol. 2018;10:299-310. doi:10.2147/CLEP.S146442
14. Oren E, Banerji A, Camargo CA Jr. Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008;121(2):533-534. doi:10.1016/j.jaci.2007.11.005
15. Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol. 2011;164(5):1078-1082. doi:10.1111/j.1365-2133.2010.10147.x
16. Solvoll K, Soyland E, Sandstad B, Drevon CA. Dietary habits among patients with atopic dermatitis. Eur J Clin Nutr. 2000;54(2):93-97. doi:10.1038/sj.ejcn.1600901
17. Huang CM, Lara-Corrales I, Pope E. Effects of vitamin D levels and supplementation on atopic dermatitis: a systematic review. Pediatr Dermatol. 2018;35(6):754-760. doi:10.1111/pde.13639
18. Bäck O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol. 2009;89(1):28-32. doi:10.2340/00015555-0541
19. Chiu YE, Havens PL, Siegel DH, et al. Serum 25-hydroxyvitamin D concentration does not correlate with atopic dermatitis severity. J Am Acad Dermatol. 2013;69(1):40-46. doi:10.1016/j.jaad.2013.01.010
20. Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for established atopic eczema. Cochrane Database Syst Rev. 2008;2008(1):CD005203. doi:10.1002/14651858.CD005203.pub2
21. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19(7):18954.
22. Okwundu N, Cardwell LA, Cline A, Unrue EL, Richardson IM, Feldman SR. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102(3):205-209.
23. Hogue L, Cardwell LA, Roach C, et al. Psoriasis and atopic dermatitis “resistant” to topical treatment responds rapidly to topical desoximetasone spray. J Cutan Med Surg. 2019;23(2):157-163. doi:10.1177/1203475418818082
24. Hindley D, Galloway G, Murray J, Gardener L. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91(2):164-168. doi:10.1136/adc.2004.050831
25. Patel N, Feldman SR. Adherence in atopic dermatitis. Adv Exp Med Biol. 2017;1027:139-159. doi:10.1007/978-3-319-64804-0_12
26. Beattie PE, Lewis-Jones MS. Parental knowledge of topical therapies in the treatment of childhood atopic dermatitis. Clin Exp Dermatol. 2003;28(5):549-553. doi:10.1046/j.1365-2230.2003.01357.x
27. Danby SG, Brown K, Wigley AM, et al. The effect of water hardness on surfactant deposition after washing and subsequent skin irritation in atopic dermatitis patients and healthy control subjects. J Invest Dermatol. 2018;138(1):68-77. doi:10.1016/j.jid.2017.08.037
28. Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13(1):15-26.
doi:10.1080/1744666X.2016.1212660
29. Chaumont A, Voisin C, Sardella A, Bernard A. Interactions between domestic water hardness, infant swimming and atopy in the development of childhood eczema. Environ Res. 2012;116:52-57. doi:10.1016/j.envres.2012.04.013
30. Mochizuki H, Tadaki H, Takami S, et al. Evaluation of out-in skin transparency using a colorimeter and food dye in patients with atopic dermatitis. Br J Dermatol. 2009;160(5):972-979. doi:10.1111/j.1365-2133.2009.09036.x
31. Papier A, Strowd LC. Atopic dermatitis: a review of topical nonsteroid therapy. Drugs Context. 2018;7:212521. doi:10.7573/dic.212521
32. Chong M, Fonacier L. Treatment of eczema: corticosteroids and beyond. Clin Rev Allergy Immunol. 2016;51(3):249-262. doi:10.1007/s12016-015-8486-7
33. Mesquita Kde C, Igreja AC, Costa IM. Atopic dermatitis and vitamin D: facts and controversies. An Bras Dermatol. 2013;88(6):945-953. doi:10.1590/abd1806-4841.20132660
34. Sampson HA. Role of immediate food hypersensitivity in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1983;71(5):473-480. doi:10.1016/0091-6749(83)90464-5
35. Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101(3):E8. doi:10.1542/peds.101.3.e8
36. Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7(1):35-39. doi:10.1016/j.jaip.2018.11.006
37. Rowlands D, Tofte SJ, Hanifin JM. Does food allergy cause atopic dermatitis? Food challenge testing to dissociate eczematous from immediate reactions. Dermatol Ther. 2006;19(2):97-103. doi:10.1111/j.1529-8019.2006.00063.x
38. Ellwood P, Asher MI, Garcia-Marcos L, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax. 2013;68(4):351-360. doi:10.1136/thoraxjnl-2012-202285
39. Mehta AB, Nadkarni NJ, Patil SP, Godse KV, Gautam M, Agarwal S. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82(4):371-378. doi:10.4103/0378-6323.178903
40. Andersen RM, Thyssen JP, Maibach HI. The role of wet wrap therapy in skin disorders - a literature review. Acta Derm Venereol. 2015;95(8):933-939.
41. Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18(3):323-332. doi:10.1007/s40257-017-0261-5
42. Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12(2):83-93.



























