
It seems like every few months I get a few similar messages in my inbox: “Mr/Mrs So-and-so is going to have a surgery, and they are wondering what they should do with their biologic?”, “When should they stop their biologic?”, or “The patient’s surgeon wants to stop their biologic before surgery; they are wondering if this is ok?”
I have always dreaded these questions, because I feel the answers are complex and not straightforward. We have the interplay of the surgeon’s word, our opinion as the dermatologist, and the patient’s fears. On the surgeon’s side of the argument, there are concerns regarding post-procedure infections and the possibility of delayed wound healing. On our side, we are also concerned with the patient’s post-procedure risk, but we are also very much concerned with their psoriasis or psoriatic arthritis (PsA) flaring after therapy cessation. So, where does this leave us as dermatologists?
Recent Research into the Answers
From the surgeon-perspective, it is known that the risk of infection with systemic immunomodulatory agents may be compounded in patients undergoing surgery, as surgical stress has been shown to induce widespread immune suppression and reduction in cell-mediated immunity.1 Tumor necrosis factor alpha (TNF-α)-promoted angiogenesis and proliferation of endothelial cells and fibroblasts are critical components to wound healing.2 Therefore, with inhibition of TNF-α, wound healing could theoretically be delayed.
In 2017, the American College of Rheumatology/American Association of Hip and Knee Surgeons published guidelines for the perioperative management of antirheumatic medication in patients with rheumatic diseases undergoing elective total hip or total knee arthroplasty.3 The panel included rheumatologists and orthopedic surgeons, and PsA was included as one of the conditions for which recommendations were made. The committee suggested that biologic agents be withheld prior to surgery in patients undergoing elective total hip or knee arthroplasty and that the surgery should be planned at the end of the dosing cycle for the specific biologic. Specifically, they suggested surgery be scheduled the week following the first withheld dose (Table 1).
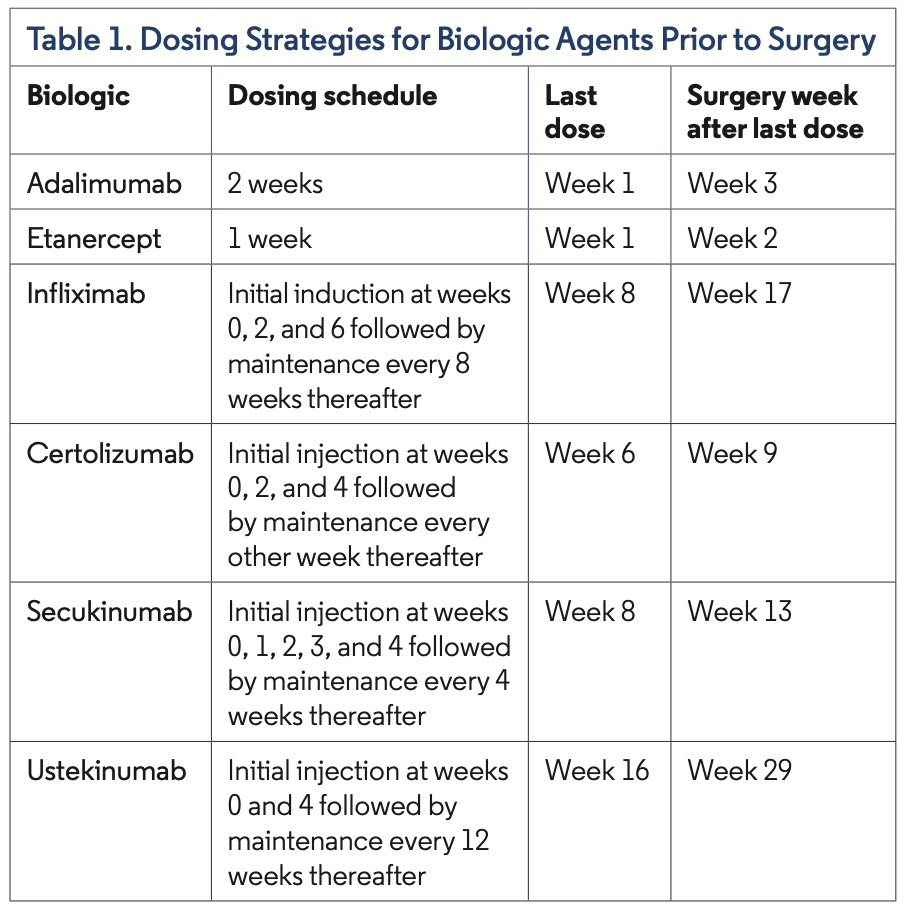
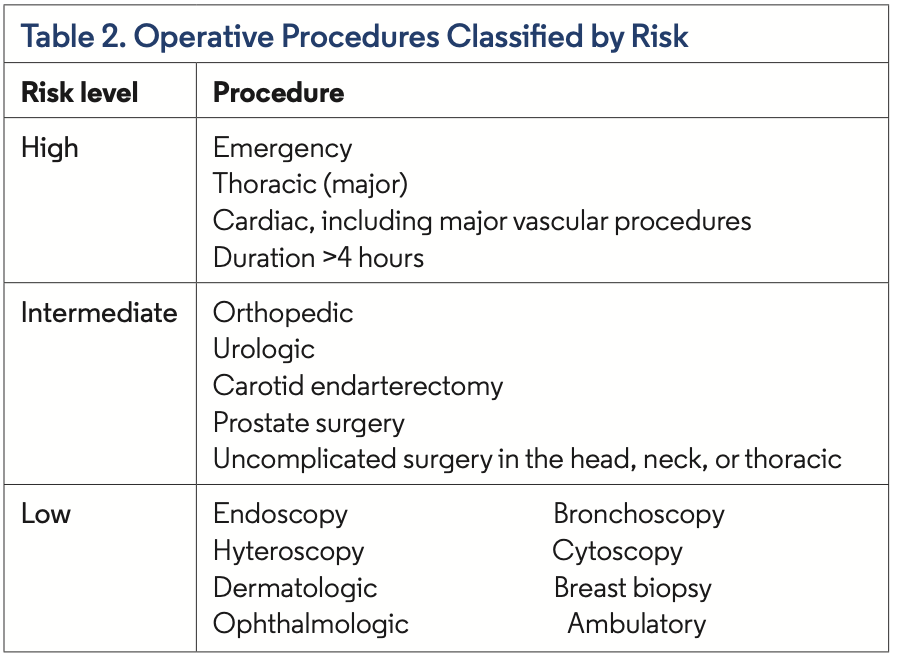
The guidelines do mention that the quality of evidence was low. There were no randomized controlled trials available in the literature, and they abstracted data from systematic reviews and meta-analyses using biologics vs placebo and in nonsurgical situations. As such, the authors deemed this to be a conditional recommendation. In addition, this recommendation was predominately based on data from rheumatoid arthritis and systemic lupus erythematosus. The commit- tee recommended that biologics could be restarted once the wound shows signs of healing (typically around 14 days), all sutures and staples are removed, and there is no significant swelling, erythema, or drainage or other clinical evidence of nonsurgical infection.3 Additionally, in 2009, the British Association of Dermatologists recom- mended withholding TNF-α inhibitors and ustekinumab for 4 half- lives before major surgery (requiring general anesthesia; Table 2).4
Overall, specific perioperative use of biologics in psoriasis data is limited. Reinstadler et al5 performed a retrospective chart review of 19 elective surgeries, including six major surgeries, in patients with psoriasis receiving TNF-α inhibitors. In five instances, the biologic was discontinued 1 to 2 weeks preoperatively, and no complications were reported. There were 14 cases in which TNF-α inhibitors were continued perioperatively. One patient undergoing a colon resection experienced a surgical site infection and prolonged wound healing, and another undergoing a root canal was documented to have a wound infection.5
A recent study6 looked at 131 patients with moderate to severe psoriasis, with 118 receiving TNF-α and 13 receiving IL-12/IL-23 inhibitors. Of the 131 patients, 58 were categorized to have a major operation and 73 minor operations. In 44 patients who discontinued their biologic at 4 half-lives before surgery, two wound infections were reported. Another 87 patients continued their biologic perioperatively, and only ove postoperative wound infection was reported.6
Bakkour et al7 performed a retrospective study of patients with psoriasis and PsA undergoing surgical procedures while on a TNF-α inhibitor or IL-12/IL-23 inhibitor. In total, 77 procedures were done with 37 categorized as major procedures. There were four cases of wound infection, two cases of delayed healing, and two cases of both wound infection and delayed healing reported. According to the authors, there were significantly more complications in cases where the TNF-α inhibitor or IL-12/IL-23 inhibitor was stopped compared with continuing the therapy through surgery (P=.14). Interestingly, a subanalysis of major surgical procedures did not find a difference in postoperative complications between continuing and stopping treatment, although the results were not statically significant (P=.253). They also found discontinuing TNF-α or IL-12/IL-23 inhibitors significantly increased the risk of psoriasis or PsA flare (P=.003).7
In 2016, the National Psoriasis Foundation Medical Board published a review of perioperative management of systemic immunomodulatory agents in patients with psoriasis and PsA.8 The authors commented that, based on the evidence they reviewed, the British Association of Dermatolgists recommendation is impractical and should be reconsidered. Notably, they added, there is sufficient level III evidence to support the safe continued use of infliximab, adalimumab, etanercept, methotrexate, and cyclosporine through low-risk operations. For intermediate and high-risk procedures, a case-by-case approach should be taken based on patient’s individual risk factors and comorbidities. They also commented there is insufficient data regarding perioperative use of certolizumab, ustekinumab, apremilast, secukinumab, and ixekizumab to make a firm recommendation.
Continuing the Conversation
As with most things in medicine, sadly there is no clear-cut answer to our questions. Although the data we have presently are not ideal and limited by the number of patients, it seems that in many situations it would be acceptable to continue the use of biologics perioperatively by taking into account each patient’s individual situation. With this knowledge, I typically will have a conversation with my patient explaining the data, recommendations, and my understanding of them. I will also pick up the phone and call my colleagues performing the surgery to discuss the data presented above and try to come to a consensus decision on what is best for the patient’s psoriatic disease along with the perioperative and postoperative risks. In doing this, I have experienced many different approaches, including some surgeons who follow the conservative recommendations mentioned previously and others who have been open to perioperative continuation. But in all situations, I have found that the patients and surgeons are appreciative of the discussion. We can only hope that there will be more data looking into these questions, including more specific data for some of the newer biologic agents (ie, IL-17 and IL-23 inhibitors) that most of us utilize with more regularity.
Dr Grosshandler practices at Westerville Dermatology in Columbus, OH, and is an associate clinical professor of dermatology at the Ohio University Heritage College of Osteopathic Medicine in Athens, OH.
References
1.Esposito S. Immune system and surgical site infection. J Chemother. 2001;13 Spec No 1(1): 12-16. doi: 10.1179/joc.2001.13.Supplement-2.12.
2.Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy. 2005;25(11):1566-1591. doi:10.1592/phco.2005.25.11.1566.
3.Goodman SM, Springer B, Guyatt G, et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the perioperative management of antirheumatic medication in patients with rheumatic diseases undergoing elective total hip or total knee arthroplasty. Arthritis Rheumatol. 2017;69(8):1538-1551. doi:10.1002/art.40149.
4.Smith CH, Anstey AV, Barker JN, et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009;161(5):987-1019. doi:10.1111/j.1365-2133.2009.09505.
5.Reinstadler A, Mau N, Bhutani T, et al. Perioperative use of anti-tumor necrosis factor –alpha agents. J Am Acad Dermatol. 2010;62(1):154-155. doi:10.1016/j.jaad.2009.04.039.
6.Fabiano A, Simone CD, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(Suppl 1):S24-S26. doi:10.1002/ddr.21189.
7.Bakkour W, Purssell H, Cinooy H, Griffiths CEM, Warren RB. The risk of post-operative complications in psoriasis and psoriatic arthritis patients on biologic therapy undergoing surgical procedures. J Eur Acad Dermatol Venereol. 2016;30(1):86-91. doi:10.1111/jdv.12997.
8.Choi YM, Debbaneh M, Weinberg JM, Yamauchi PS, Van Voorhees AS, Armstrong AW, Siegel M, Wu JJ. From the Medical Board of the National Psoriasis Foundation: Perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016 Oct;75(4):798-805.e7. doi: 10.1016/j.jaad.2016.06.014. Epub 2016 Jul 25. PMID: 27461230.


























