What The Emerging Research Reveals About Continuous Glucose Monitoring
 It has been well-established that many of the lower extremity complications caused by the diabetes mellitus disease process, primarily macro- and micro-vascular disease, are minimized in the setting of tight long-term glycemic control as measured by the glycated hemoglobin (HbA1c) value.
It has been well-established that many of the lower extremity complications caused by the diabetes mellitus disease process, primarily macro- and micro-vascular disease, are minimized in the setting of tight long-term glycemic control as measured by the glycated hemoglobin (HbA1c) value.
Although studies such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the 1993 study by the Diabetic Control and Complications Trial Research Group (DCCT), the United Kingdom Prospective Diabetes Study (UKPDS) and Veterans Affairs Diabetes Trial (VADT) have demonstrated the substantial impact of lower glycated hemoglobin values in reducing the vascular complications caused by diabetes, they also demonstrate that HbA1c values less than 7 percent might result in a greater frequency of adverse outcomes.1-4 The American Diabetes Association (ADA) defines a glycated hemoglobin value of 7 percent as “well-controlled.”5 However, particularly in elderly patients and brittle patients with diabetes, tight regulation is likely to result in more frequent and more severe hypoglycemic episodes.
These are dangerous events and potentially underappreciated by podiatric physicians. These events often result in hospitalization, coma, seizures and even death.6 Shorr and colleagues found that the greatest predictors of hypoglycemia development in patients with diabetes were advanced age, recent hospitalization and polypharmacy.6 The ACCORD study reported that those patients in an intensive glycemic control therapy group (HbA1c goal <6 percent) experienced three times the rate of hypoglycemia in comparison to those in a standard glycemic control therapy group (HbA1c goal 7-7.9 percent).1 Further, participants greater than 65 years of age experienced twice the number of hypoglycemic episodes in comparison to younger patients.7
Due to this increased risk of hypoglycemia in elderly patients, Lipska and coworkers have recommended individualized treatment plans for elderly patients with an emphasis on avoiding abnormally low glucose levels.8 Although those with brittle diabetes may experience many hypo- and hyperglycemic episodes throughout the day, this variability might be masked by a seemingly normal HbA1c level.9,10 This glycemic variability may cause increased oxidative stress and result in long-term complications aside from those caused by chronic hyperglycemia.11
How Continuous Glucose Monitoring Works
 These potential issues might be better controlled through the development of technology associated with continuous glucose monitoring. Many of these devices are connected to smartphone applications in order to allow the patient immediate and objective feedback on glucose trends throughout the day. The patient’s primary care physician or endocrinologist can further utilize this information to help develop personalized regimens.
These potential issues might be better controlled through the development of technology associated with continuous glucose monitoring. Many of these devices are connected to smartphone applications in order to allow the patient immediate and objective feedback on glucose trends throughout the day. The patient’s primary care physician or endocrinologist can further utilize this information to help develop personalized regimens.
Additionally, many glucose monitoring units are equipped with alarms to alert patients of rapidly falling or rising glucose levels, which can be specifically useful in detecting nocturnal hypoglycemia when finger checks are not routine. This might be very important as many patients are unaware of their hypoglycemic episodes.12 For example, a recent study evaluated a continuous glucose monitor for five days in 108 patients with type 2 diabetes.13 The authors found that 49.1 percent of patients had at least one hypoglycemic episode but 75 percent of these patients were asymptomatic with respect to the episode.
Impaired hypoglycemia awareness is a risk factor for hypoglycemic events. In a study by Schopman and colleagues, a group of patients with type 1 diabetes and impaired hypoglycemia awareness experienced twice the number of hypoglycemic episodes in comparison to those with normal awareness, and had seven times the number of asymptomatic hypoglycemic episodes.14 Although most studies have focused on the use of continuous glucose monitors in lowering A1c, these monitors may have a role in preventing these hypoglycemic episodes.15
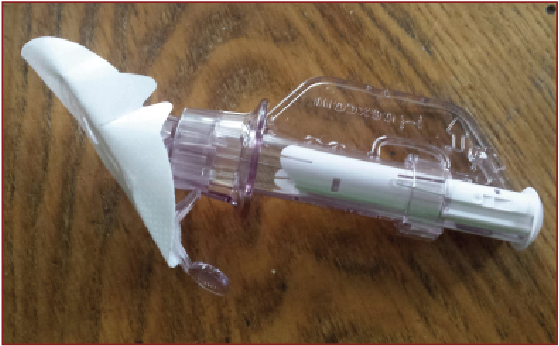
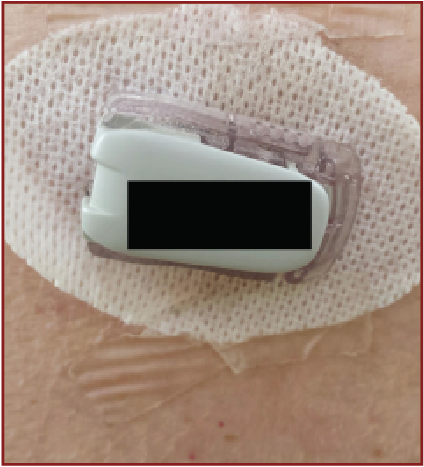
When operating a glucose monitor, one would first place a small needle sensor into the subcutaneous tissue, usually of either the abdomen or back, with a plunger. Then connect a transmitter to the needle and secure the entire construct with adhesive. One must replace the needle approximately once a week and also clean the transmitter at that time. While it is in place, the unit is waterproof and portable. A handheld receiver (or a smartphone application) then provides updates of glucose levels every few minutes. Both the patient and the physician can utilize this information to monitor trends and adjust therapies as needed.
An online questionnaire to 877 users of a continuous glucose monitor found that 86 percent of patients reported improvement in their perceived control over diabetes, 78 percent reported less fear about hypoglycemia and 79.9 percent stated that they felt safe while sleeping.16
 It is still recommended that patients perform finger-stick measurements in addition to using the monitor. Part of this is because the monitor must be calibrated approximately every 12 hours with a self-monitored blood glucose meter measurement. Further, there are slight differences in blood glucose measurements between those detected by the finger-stick measurement and the subcutaneous needle of the continuous glucose monitor. The later relies on interstitial fluid and there is a time delay as glucose transports across blood vessel walls from the plasma into the interstitial fluid. This might result in a “sensor lag” or a delay between the actual blood glucose and the sensor’s ability to detect it. As an example of this, Ward and colleagues found an approximate 1.5-minute delay behind falling glucose levels with use of a continuous glucose monitor.17
It is still recommended that patients perform finger-stick measurements in addition to using the monitor. Part of this is because the monitor must be calibrated approximately every 12 hours with a self-monitored blood glucose meter measurement. Further, there are slight differences in blood glucose measurements between those detected by the finger-stick measurement and the subcutaneous needle of the continuous glucose monitor. The later relies on interstitial fluid and there is a time delay as glucose transports across blood vessel walls from the plasma into the interstitial fluid. This might result in a “sensor lag” or a delay between the actual blood glucose and the sensor’s ability to detect it. As an example of this, Ward and colleagues found an approximate 1.5-minute delay behind falling glucose levels with use of a continuous glucose monitor.17
A Caution On The Limitations Of Continuous Glucose Monitoring
Although continuous glucose monitors have been available for over 10 years, very few patients with diabetes actually utilize them. This is due to some limitations associated with the technology. One of these is the out-of-pocket cost to the patient if the product does not get reimbursement. In a 2008 study, Halford and Harris assessed self-reported satisfaction rates among 54 patients who had used continuous glucose monitors. Of those who stopped using the device, 93 percent cited “cost” as one of the determining factors with 30 percent of those people listing it as the sole reason for discontinuation.18 Only 33 percent of responders said they had issues with the adhesive and 30 percent said the device was too bulky. In the coming years, we’ll likely see cost analyses that look at the cost of a hypoglycemic hospitalization in comparison to the cost of a continuous glucose monitoring unit.
Additionally, it is still unclear which specific patient populations would benefit most from a continuous glucose monitor although these populations are most likely elderly and patients with type 2 diabetes. Further, analyzing the data provided from monitors takes a substantial amount of time that physicians may not have available. Although the continuous glucose monitor was expected to be the long-awaited answer to prevention of severe hypoglycemia, researchers have not clearly demonstrated this in clinical studies, in part due to underpowered samples and study design.15,19
In Conclusion
Hopefully, in the future, this technology will become more readily available to patients and have improved functionality. The holy grail may be a “smart system,” which simultaneously monitors glucose levels and automatically adjusts insulin infusion rates accordingly.20 This ability to control glucose within a normal range would be “the next best thing to a cure for diabetes.”20 Potential improvements in the monitors include coated needles that will reduce lower accuracy toward the end of their life cycles. This may also reduce the “run-in time,” which is the time immediately following needle insertion during which readings are most inaccurate. Most importantly, there needs to be further clinical trials demonstrating the effectiveness of continuous glucose monitors and which populations would benefit most from them.
On a personal note, the lead author’s grandfather has been an insulin-dependent patient with diabetes for 30 years and is 80 years old. He has been using a continuous glucose monitor for approximately three months. His endocrinologist has set his goal A1c between 8 to 9 percent and his monitor is programmed to alert him at <100 mg/dL and >400 mg/dL. He has had multiple hospitalizations for low blood glucose in the past, due in part to the fact that he still tends to a 70-acre horse farm. Insurance does not cover his monitoring unit and the start-up cost was approximately $1,200. Further, it costs him approximately $100 to replace the needle every week. He believes the readings have been accurate when he has checked his glucose against his monitor and it has awoken him from sleep approximately once a week with a hypoglycemic warning.
Dr. Crowell is the Chief Resident within the Temple University Hospital Podiatric Surgical Residency Program in Philadelphia.
Dr. Meyr is a Clinical Associate Professor and Residency Program Director in the Department of Podiatric Surgery at Temple University School of Podiatric Medicine and Temple University Hospital in Philadelphia.
References
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358(24):2545-59.
- No authors listed. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329(14):977-86.
- No authors listed. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352(9131):837-53.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360(2):129-39.
- American Diabetes Association. Standards of medical care in diabetes – 2016. Diabetes Care. 2016; 39(Suppl 1):S1-S106.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonulureas. Arch Intern Med. 1997; 157(15): 1681-6.
- Wong JC, Foster NC, Maahs DM, et al. T1D Exchange Clinic Network. Diabetes Care. 2014; 37(10):2702-9.
- Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care. 2013; 36(11):3535-42.
- De Block C, Manuel-y-Keenoy B, Van Gaal L, Rogiers P. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care. 2006; 29(8):1750-6.
- De Block C, Manuel-y-Keenoy B, Rogiers P, et al. Glucose control and use of continuous glucose monitoring in the intensive care unit: a critical review. Curr Diabetes Rev. 2008; 4(3): 234-44.
- Ceriello A, Novials A, Ortega E, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes. 2012; 51(11):2993-7.
- Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than thresholds for symptoms. J Clin Invest. 1987; 79(3):777-81.
- Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia and type 2 diabetes—more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol. 2015; 9(5):999-1005.
- Schopman JE, Geddes J, Frier BM. Frequency of symptomatic and asymptomatic hypoglycaemia in type 1 diabetes: effect of impaired awareness of hypoglycaemia. Diabet Med. 2011; 28(3):352-5.
- Van Beers CA, Kleijer SJ, Serne EH, Geelhoed-Dujivestijn PH, Snoek FJ, Kramer MH, Diamant M. Design and rationale of the IN CONTROL trial: the effects of real-time continuous glucose monitoring on glycemia and quality of life in patients with type 1 diabetes mellitus and impaired awareness of hypoglycemia. BMC Endocr Disord. 2015; 15:42.
- Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013; 15(4):295-301.
- Ward WK, Engle JM, Branigan D, El Youssef J, Massoud RG, Castle JR. The effect of rising vs. falling glucose level on amperometric glucose sensor lag and accuracy in type 1 diabetes. Diabet Med. 2012; 29(8):1067-73.
- Halford J, Harris C. Determining clinical and psychological benefits and barriers with continuous glucose monitoring therapy. Diabetes Technol Ther. 2010; 12(3):201-5.
- Wentholt IM, Hoekstra JB, Devries JH. Continuous glucose monitors: the long-awaited watch dogs? Diabetes Technol Ther. 2007; 9(5):399-409.
- Lodwig V, Kulzer B, Schnell O, Heinemann L. What are the next steps in continuous glucose monitoring? J Diabetes Sci Technol. 2014; 8(2):397-402.











