Podiatrists play a critical role in the identification, treatment and management of patients with peripheral arterial disease (PAD). While an estimated 8 to 12 million Americans have PAD, the disease continues to remain largely underdiagnosed.
1 Symptoms of pain, aching or cramping in the legs with walking (claudication) can occur in the buttock, hip, thigh, or calf.
1 However, up to 75 percent of individuals with PAD are either asymptomatic, unaware of the signs of PAD or have symptoms that are not appropriately identified by their primary healthcare provider.
2-5 Those with diabetes and neuropathy may not have any of the classic symptoms and instead complain only of lower extremity fatigue that rest relieves.
With the demographic changes in Western societies trending to older, more obese people prone to type 2 diabetes and hypertension, there is increasing interest in the comorbid effects of PAD and diabetes on the cardiovascular system. The standard estimates of prevalence of lower extremity PAD based on ankle-brachial index blood pressure ratios are approximately 10 to 20 percent of community-dwelling individuals aged 65 and older and 18 to 29 percent of patients aged 50 and older in general medicine practices.
6-9 Individuals with diabetes have a much higher risk for PAD with a 1 in 3 chance of developing the disease. The risk of PAD increases four times for people who smoke or have a history of smoking. Consequently, PAD within these patient populations requires early disease identification, a structured treatment plan and close patient monitoring in order to avoid complex surgical procedures, critical limb ischemia and amputation.
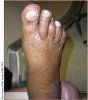
Unfortunately, awareness of PAD symptoms almost always occurs first with issues on the patient’s foot as the cumulative effects of diabetes and diseased arterial flow result in a cascade of events that include loss of protective sensation, toe/foot deformities, tissue breakdown, non-healing ulcers and ultimately gangrene.
Recognizing The Pros And Cons Of The Ankle Brachial Index
The most common frontline test to assess for the presence of PAD is the ankle brachial index (ABI). One obtains the ABI by measuring the systolic pressures at the brachial arteries and comparing these to the systolic pressures at the dorsalis pedis (anterior tibial) and posterior tibial arteries. For many patients, the ABI exam is a reliable and accurate PAD indicator. While the United States Preventive Services Task Force has never endorsed ABI for use as a screening tool for PAD or cardiovascular disease, all professional societies endorse the use of ABI in patients who are positive for risk indicators and signs/symptoms.
10
However, the ABI is not without its limitations. Tibial artery disease occurs at a much higher rate in patients with diabetes in comparison to patients without diabetes.
11 As part of the complex disease process, patients with diabetes often develop medial calcinosis in the tibial arteries, stiffening arterial walls. The significance of these less compliant or compression resistant arteries related to the ABI test is that when taking pressures at the ankle, it often requires increased cuff pressure to stop arterial flow, resulting in an artificially normal or high ABI. When comparing the compression resistant tibial artery pressures to the brachial artery pressures to calculate the index, one can find an artificially elevated ABI resulting in a misdiagnosis or underdiagnosis of the patient’s true lower extremity arterial condition.
12,13 The ABI may also be falsely normal in symptomatic patients with moderate aortoiliac stenosis.
11
Contraindications to ABI are documented in the Society for Vascular Ultrasound’s Lower Extremity Arterial Duplex Evaluation guidelines.
11 These contraindications to ABI include:
• patients with suspected or known acute deep venous thrombosis (DVT) or superficial thrombophlebitis;
• those who have had recent surgery, ulcers, casts or bandages that cannot or should not be compressed by pressure cuffs;
• patients with incompressible arteries due to medial calcification;
• patients who have had an interventional procedure (i.e., stent placement, arterial bypass graft); and
• patients who have had a lower extremity (ankle level) interventional procedure (i.e., ankle level arterial bypass graft).
When PVR And SPP Testing Can Have An Impact
With these caveats in mind, patients with combination disease require tests that are not subject to the same limitations as ABI but that are also conducive to outpatient, clinic-based vascular testing. Two tests that meet these criteria include air or volume plethysmography (also called pulse volume recording or PVR) and the skin perfusion pressure (SPP) test, which measures reactive hyperemia.
The PVR test requires the use of pressure cuffs that one can place at various levels. With each cardiac cycle, blood pumps throughout arteries. The PVR test uses a partially inflated pressure cuff to apply slight pressure to the limb. The impact of blood passing through the limb transfers to the pressure cuff where the cuff measures the impact as small changes in cuff pressure. The changes display as a PVR waveform. The amplitude and shape (pulse contour) of these waves provide an indication of blood volume in the limb arteries. The normal pulse volume recording is composed of a systolic upstroke with a sharp systolic peak followed by a downstroke that contains a prominent dicrotic notch. Changes in the pulse volume contour such as disappearance of the dicrotic notch and loss of a sharp systolic peak indicate proximal arterial obstruction and are due to the dissipated energy that occurs due to arterial narrowing. These changes can be a sign of PAD. One must perform bilateral PVRs in order to compare them to each other since disease can be different in both legs.
15
Skin perfusion pressure, a provocative functional test of the capillaries, is a measurement of reactive hyperemia. For the SPP test, the clinician inflates a circumferential cuff to a known pressure and holds it at that pressure for a specified period of time. This subjects the capillaries to a brief period of pressure-induced ischemia. When one deflates the pressure cuff under an automated release protocol, reactive hyperemia occurs. The cuff pressure (mmHg) when the capillary bed reperfuses (hyperemia) is the skin perfusion pressure.
A simple way to think of skin perfusion pressure is capillary blood pressure. To perform the test, one would place a laser Doppler probe on the patient. Light from the laser enters the tissue and interacts with tissue and capillaries. The probe assesses light that returns through the laser to the instrument for the Doppler signal. The probe processes this signal and translates it into the cuff pressure at which flow was able to return to the capillary beds. In normal, healthy adults, skin perfusion pressure ranges are 70 to 110 mmHg.
16-18 Perfusion pressures associated with PAD are typically in the range of 30 to 60 mmHg while critical limb ischemia is defined as perfusion pressures below 30 mmHg.
Outside of PAD detection and management, SPP/PVR/ABI testing also provides valuable vascular information to guide many of the common treatment decisions facing the podiatrist regarding the diabetic foot. Not only is adequate tissue perfusion required to heal a diabetic foot ulcer but in cases of elective, prophylactic or curative surgery, one should assess perfusion in order to predict wound healing.
Myth: ‘An ABI Is An ABI’
In October 2012, the American Heart Association (AHA) published a scientific statement regarding the measurement and interpretation of ABI.
19 The AHA Writing Committee’s review of the literature found multiple variations in technique for measuring and interpreting ABI, including different positions for the patient during measurement, different sizes of the arm and leg cuffs, different locations of the cuff on the extremity, and different methods of pulse detection.
Also, the committee found that not all physicians use the same ABI thresholds to diagnose peripheral disease. The Writing Committee disclosed that it is not commonly understood that ABI requires one to measure two separate systolic arterial (dorsalis pedis and posterior tibial) pressures at each ankle and the systolic brachial arterial pressures in both arms in order to compare the pressures in what becomes a comparative ratio. Specifically, the committee reviewed two methods used to report ABI and the literature showing that these methods had more significant clinical implications than are generally realized.
The Doppler method to calculate the ABI requires the use of continuous-wave Doppler probe for the detection of arterial flow. The clinician puts the probe into position over the artery of interest (brachial, dorsalis pedis (DP), posterior tibial (PT)). One then applies a pneumatic pressure cuff to the upper arm for the brachial assessment or to the ankle for the dorsalis pedis and posterior tibial assessment. Inflate the cuff to 20 mmHg above the pressure wherein blood flow stops and then deflate it slowly until there is reappearance of the Doppler flow signal (visual and/or audio). The corresponding cuff pressure is the systolic blood pressure. One records each of the three pressures bilaterally and uses them to calculate the ABI. The calculation for ABI requires the clinician to divide the higher of the posterior tibial or dorsalis pedis pressure by the higher of the arm systolic blood pressure, and report this ratio separately for each leg in patients with symptoms of PAD.
The Writing Committee also addressed other methods of performing ABI tests and these methods included the use of plethysmography, photoplethysmography, auscultation, and pulse palpation. The committee concluded that none were acceptable alternatives to Doppler ABI in terms of reproducibility, specificity and/or sensitivity, and that clinicians should not use them for clinical decision-making.
What You Should Know About The Medicare Requirements
As of January 1, 2011, Medicare issued the CPT code language for non-invasive physiologic peripheral artery tests (CPT code 93922/93923). The code underwent a significant change from previous language. These changes were intended to reflect the considerable clinical evidence relative to best practice when using ABI as part of the diagnostic array that is required to detect and manage PAD. To that end, the 2011 language identifies the specific methods, levels and arterial assessment locations required to generate a reimbursable ABI as part of an overall lower extremity arterial assessment. With the 2012 AHA Scientific Statement, these changes are fully explained and validated in terms of published evidence.
19
The CPT 2011 code makes it clear that the Doppler method is required to perform an ABI test that meets the Medicare requirement for reimbursement eligibility using CPT Codes 93922-93924. In addition to the CPT 2011 language change, several regional Medicare carriers revised the language in their local coverage determinations to specifically identify non-covered methods such as oscillometry or photoplethysmography.
Clinicians would use CPT code 93922 when performing a “limited” arterial study involving bilateral assessments on one to two levels on the lower extremity. One would employ CPT code 93923 when performing “complete” arterial study involving bilateral assessments on three or more levels on the lower extremity. The CPT code 93923 is also appropriate for a single level study with provocative functional maneuvers (i.e. reactive hyperemia).
For more information on the Medicare requirements for non-invasive physiologic arterial testing, you should contact your regional Medicare provider.
In Conclusion
Podiatrists play a key role in the detection, treatment and management of patients with PAD. Incorporating patient awareness initiatives, performing diagnostic assessments and utilizing the optimal testing methods will provide optimal outcomes.
Dr. Rogers is the Medical Director of the Amputation Prevention Center at the Sherman Oaks Hospital in Sherman Oaks, Ca. He is an Assistant Professor of Podiatry at the Western University of Health Sciences in Pomona, Ca.
References
1. Rooke TW, Wennberg PW. Diagnosis and management of diseases of the peripheral arteries and veins. In: Walsh RA, Simon DI, Hoit BD, et al. (eds):
Hurst’s The Heart, 12th ed., McGraw Hill, New York, 2007.
2. Hirsh AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care.
JAMA. 2001;286(11):1317-1324
3. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics — 2011 update: a report from the American Heart Association.
Circulation. 2011;123(4):e18-e209.
4. McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease.
Arch Intern Med. 1999;159(4):387–392.
5. United States Preventive Services Task Force. Screening for peripheral arterial disease: a brief evidence update for the U.S. Preventive Services Task Force (USPSTF). Available at www.uspreventiveservicestaskforce.org/uspstf05/pad/padup.htm . Published August 2005. Accessed May 21, 2013.
6. Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population.
Int J Epidemiol. 1991;20(2):384-92.
7. Goldman L, Bennett JC (eds).
Cecil's Textbook of Medicine, 21st ed., W.B. Saunders Company, Philadelphia, 2000.
8. Schroll M, Munck O. Estimation of peripheral arteriosclerotic disease by ankle blood pressure measurements in a population study of 60 year-old men and women.
J Chronic Dis. 1981;34(6):261-9.
9. Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the Rotterdam study.
Arterioscler Thromb Vasc Biol. 1998;18(2):185-92
10. U.S. Preventive Services Task Force. U.S. Preventive Services Task Force issues draft recommendation statement: screening for peripheral artery disease and cardiovascular risk assessment with ankle brachial index. Available at www.uspreventiveservicestaskforce.org/bulletins/padbulletin.pdf . Published March 19, 2013. Accessed April 23, 2014.
11. American Diabetes Association. Peripheral arterial disease in people with diabetes.
Clinical Diabetes. October 2004;22(4):181-189
12. Smith DC, Bilmen GJ, Igbal S, Robey S, Pereira M. Medial artery calcification as an indicator of diabetic peripheral vascular disease.
Foot Ankle Int. 2008;29(2):185-190.
13. Aerden D, Massaad D, von Kemp K, van Tussenbroek F, Debing E, Keymeulen B, et al. The ankle-brachial index and the diabetic foot: a troublesome marriage.
Ann Vasc Surg. 2011;25(6):770-777.
14. Society for Vascular Ultrasound. Lower extremity arterial duplex evaluation guidelines. Available at www.svunet.org/practicemanagement/professionalperformanceguidelines . Published August 10, 2012. Accessed April 23, 2014.
15. Kempczinski RF. Segmental volume plethysmography in the diagnosis of lower extremity arterial occlusive disease.
J Cardiovasc Surg. 1982;23(2):125-9.
16. Hatakeyama S, Saito M, Ishigaki K, Yamamoto H, Okamoto A, Ishibashi Y, et al. Skin perfusion pressure is a prognostic factor in hemodialysis patients.
Int J Nephrol. 2012;2012:385274. Doi: 10.1155/2012/385274. Epub April 2, 2012.
17. Kanetaka T, Komiyama T, Onozuka A, Miyata T, Shigematsu H. Laser Doppler skin perfusion pressure in the assessment of Raynaud’s phenomenon.
Eur J Vasc Endovasc Surg. 2004;27(4):414-416.
18. Kanai et al. Assessment of upper limb circulation in hemodialysis patients with laser Doppler skin perfusion pressure; Influences of vascular access, 2009 World Congress Nephrology Abstracts, Milan, Italy, May 22-26
19. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager M, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association.
Circulation 2012;126(24):2890-909.
Editor’s note: For further reading, see “Current Insights On ABI And Diagnosing PAD” in the September 2013 issue of Podiatry Today, “How To Diagnose Peripheral Arterial Disease” in the April 2007 issue or “Keys To Diagnosing And Addressing PAD In Patients With Wounds” in the March 2011 issue.
For other related articles, visit the archives at www.podiatrytoday.com
 With the demographic changes in Western societies trending to older, more obese people prone to type 2 diabetes and hypertension, there is increasing interest in the comorbid effects of PAD and diabetes on the cardiovascular system. The standard estimates of prevalence of lower extremity PAD based on ankle-brachial index blood pressure ratios are approximately 10 to 20 percent of community-dwelling individuals aged 65 and older and 18 to 29 percent of patients aged 50 and older in general medicine practices.6-9 Individuals with diabetes have a much higher risk for PAD with a 1 in 3 chance of developing the disease. The risk of PAD increases four times for people who smoke or have a history of smoking. Consequently, PAD within these patient populations requires early disease identification, a structured treatment plan and close patient monitoring in order to avoid complex surgical procedures, critical limb ischemia and amputation.
Unfortunately, awareness of PAD symptoms almost always occurs first with issues on the patient’s foot as the cumulative effects of diabetes and diseased arterial flow result in a cascade of events that include loss of protective sensation, toe/foot deformities, tissue breakdown, non-healing ulcers and ultimately gangrene.
With the demographic changes in Western societies trending to older, more obese people prone to type 2 diabetes and hypertension, there is increasing interest in the comorbid effects of PAD and diabetes on the cardiovascular system. The standard estimates of prevalence of lower extremity PAD based on ankle-brachial index blood pressure ratios are approximately 10 to 20 percent of community-dwelling individuals aged 65 and older and 18 to 29 percent of patients aged 50 and older in general medicine practices.6-9 Individuals with diabetes have a much higher risk for PAD with a 1 in 3 chance of developing the disease. The risk of PAD increases four times for people who smoke or have a history of smoking. Consequently, PAD within these patient populations requires early disease identification, a structured treatment plan and close patient monitoring in order to avoid complex surgical procedures, critical limb ischemia and amputation.
Unfortunately, awareness of PAD symptoms almost always occurs first with issues on the patient’s foot as the cumulative effects of diabetes and diseased arterial flow result in a cascade of events that include loss of protective sensation, toe/foot deformities, tissue breakdown, non-healing ulcers and ultimately gangrene.
 With the demographic changes in Western societies trending to older, more obese people prone to type 2 diabetes and hypertension, there is increasing interest in the comorbid effects of PAD and diabetes on the cardiovascular system. The standard estimates of prevalence of lower extremity PAD based on ankle-brachial index blood pressure ratios are approximately 10 to 20 percent of community-dwelling individuals aged 65 and older and 18 to 29 percent of patients aged 50 and older in general medicine practices.6-9 Individuals with diabetes have a much higher risk for PAD with a 1 in 3 chance of developing the disease. The risk of PAD increases four times for people who smoke or have a history of smoking. Consequently, PAD within these patient populations requires early disease identification, a structured treatment plan and close patient monitoring in order to avoid complex surgical procedures, critical limb ischemia and amputation.
Unfortunately, awareness of PAD symptoms almost always occurs first with issues on the patient’s foot as the cumulative effects of diabetes and diseased arterial flow result in a cascade of events that include loss of protective sensation, toe/foot deformities, tissue breakdown, non-healing ulcers and ultimately gangrene.
With the demographic changes in Western societies trending to older, more obese people prone to type 2 diabetes and hypertension, there is increasing interest in the comorbid effects of PAD and diabetes on the cardiovascular system. The standard estimates of prevalence of lower extremity PAD based on ankle-brachial index blood pressure ratios are approximately 10 to 20 percent of community-dwelling individuals aged 65 and older and 18 to 29 percent of patients aged 50 and older in general medicine practices.6-9 Individuals with diabetes have a much higher risk for PAD with a 1 in 3 chance of developing the disease. The risk of PAD increases four times for people who smoke or have a history of smoking. Consequently, PAD within these patient populations requires early disease identification, a structured treatment plan and close patient monitoring in order to avoid complex surgical procedures, critical limb ischemia and amputation.
Unfortunately, awareness of PAD symptoms almost always occurs first with issues on the patient’s foot as the cumulative effects of diabetes and diseased arterial flow result in a cascade of events that include loss of protective sensation, toe/foot deformities, tissue breakdown, non-healing ulcers and ultimately gangrene.











