ADVERTISEMENT
New Products March 2019
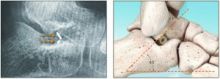 Flatfoot Correction At Its Peak
Flatfoot Correction At Its Peak
A new implant may provide podiatric surgeons with an alternative option for correcting progressive flatfoot in adult and pediatric patients.
The PitStop™ Implant is made from polyether ether ketone (PEEK), a high-performance polymer, which can facilitate long-term patient tolerance, notes the manufacturer In2Bones. The implant can reportedly help restore stability to the arch during ambulation, according to the company.
As In2Bones says, traditional metallic implants have limits due to the excessive stiffness of the material, which can cause pain and biomechanical adaptations. The company says the PEEK in PitStop is less stiff than metal and its elasticity is more similar to bone.
Company: In2Bones
Product: PitStop™ Implant
For more info, go to www.PitStop-Implant.com .
 Injectable Bone Augmentation
Injectable Bone Augmentation
A growth factor for bone is now available in an injectable form.
Augment Injectable is composed of recombinant human platelet-derived growth factor (rhPDGF-BB), notes the manufacturer Wright Medical Technologies. The product also contains beta-tricalcium phosphate (β-TCP), a substance similar to bone. The company adds that a collagen matrix makes the Augment Injectable flowable.
Wright Medical notes that Augment is the first and only Food and Drug Administration (FDA)-approved alternative to autografts in ankle and hindfoot arthrodesis. The company says the injectable delivers necessary bone growth potential for hindfoot and ankle fusion procedures, while obviating the necessity for autografts and the morbidities associated with autograft harvest.
Company: Wright Medical Technologies
Product: Augment Injectable
For more info, go to www.augmentbonegraft.com/ .
A Redesigned Cannulated Screw
The manufacturers of an established cannulated screw have recently updated the product for foot and ankle surgeons.
The Mecron® Cannulated Screw is made from a titanium alloy and is available in headed and headless forms, notes the manufacturer Merete Technologies. The screws come in diameters from 2.0 mm through 4.0 mm and in overall lengths from 8 mm through 50 mm.
The company notes the Mecron Screw can provide optimal compression and facilitate precise tracking. Merete adds that the screw’s reverse cutting flutes aid in easy screw removal.
Company: Merete Technologies
Product: Mecron® Cannulated Screw
For more info, go to www.mereteusa.com/details/mecron-cannulated-screws/.
Providing Bone Stability Without A Trace
An innovative bone pin may provide enhanced stability to help faciliate smoother post-op healing after lower extremity surgery.
The OSSIOfiber™ Bone Pin Family, which recently received FDA 510(k) market clearance, utilizes a proprietary bio-integrative material to provide stability and maintain alignment following osteotomies, arthrodesis and bone grafts, notes the manufacturer Ossio. The company says the implant’s natural mineral fiber matrix is biologically friendly, gradually transferring the load to native bone and leaving no permanent hardware behind.
Ossio notes that OSSIOfiber features easy insertion and secure fixation with a mechanical strength that is significantly higher than cortical bone. The company adds that the implant’s stiffness is a better mechanical match for bone, which can prevent stress risers and weakening of the bone around the implant. Citing pre-clinical studies, the company says the OSSIOfiber’s full integration into the surrounding anatomy takes about 18 to 24 months.
Company: Ossio
Product: OSSIOfiber™ Bone Pin Family
For more info, go to www.ossio.io.











