ADVERTISEMENT
New Products July 2019
Find out more about new product releases and emerging technology this month.
New NPWT Drape Conforms For Optimal Seal
A silicone-acrylic hybrid negative pressure wound therapy (NPWT) drape was recently granted Food and Drug Administration (FDA) 510(k) clearance as an accessory to certain KCI NPWT systems.
DERMATAC™ drape conforms to the anatomic location to provide a tight seal for 48 to 72 hours, including uneven areas, according to the manufacturer KCI. The company cites enhanced functionality in comparison to other drapes and faster dressing changes in comparison to the standard of care.
The drape also allows for repositioning during initial application, according to the company. KCI also notes that patients are able to shower and ambulate, if indicated, due to the combination of materials in the product.
Company: KCI
Product: DERMATAC™ drape
For more info, go to https://mykci.com/products/dermatac-drape
A Three-Dimensional Orthotic Scanning System
Footmaxx has introduced 3Dmaxx, a three-dimensional volumetric foot scanning device. Clinicians can use 3Dmaxx alone or in combination with the company’s Metascan system for dynamic gait analysis capabilities, according to Footmaxx.
Physicians can access the web-based software from any location, and can cast in non-weightbearing, semi-weightbearing or weightbearing positions, notes the company.
Footmaxx says the 3DMaxx scan completes in as fast as eight seconds with resolution of 250 micrometers and up to 800,000 points of data. The system allows for a 14” by 16” field of view and variable positioning. Footmaxx also notes scanning requires a USB connection to a computer with a Windows-based operating system.
Company: Footmaxx
Product: 3Dmaxx
For more info, go to www.footmaxx.com or www.3dmaxx.com.
A New Instrumentation Set For Endoscopic Soft Tissue Release
A new surgical instrumentation system may provide podiatric surgeons with an alternative when performing endoscopic soft tissue releases. ClearGuard LE™ is used exclusively in the lower extremity and has applications for tarsal tunnel and plantar fascial releases, gastrocnemius recession, and Morton’s neuroma, notes In2Bones.
The system allows for panoramic visualization of the surgical site with a transparent cannula, minimized incision and less tissue disruption. The company cites a proprietary “guided track” technology that allows the surgeon to independently operate the arthroscope and blade from within the cannula. Safety features of the cannula include a blocked endpoint for additional control.
ClearGuard LE has universal scope compatibility, is provided sterile and is disposable after a single use. The set includes the ClearGuard LE cannula, forward-cutting blade, in-situ rasp, synovial elevator/rasp and sequential dilators.
Company: In2Bones
Product: ClearGuard LE™
For more info, go to https://i2b-usa.com/clearguard-le/.
Diverse Laser Gets FDA Clearance
The Remy, a class IV laser, recently obtained FDA clearance, according to Zuckerman Future Technologies. This laser is cleared for treatment of pain and/or inflammation associated with conditions such as Achilles tendonitis and plantar fasciitis, according to the company. The company adds that the laser is also cleared for surgical procedures including wart removal and toenail micro-drilling.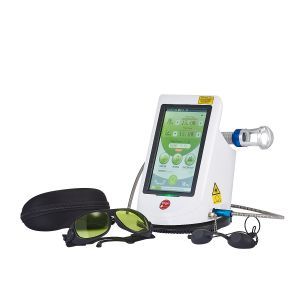
Clinicians can customize use of the Remy with four wavelengths. Weighing only four pounds, it has a seven millimeter handpiece with a finger-switch feature. This allows physicians to change from pain to fungal treatment in seconds without the need for a foot pedal, according to the manufscturer.
Zuckerman Future Technologies is a direct importer of the Remy class IV laser technology.
Company: Zuckerman Future Technologies
Product: Remy Class IV Laser
For more info, go to https://www.zuckermanft.com/class-iv-laser/.











