A Guide To Dermatological Side Effects Of Chemotherapy And Targeted Cancer Therapy
Podiatrists can play a vital role in the overall outcomes and quality of life for patients undergoing treatment for cancer. Here the authors outline several dermatologic side effects that patients may experience as part of their cancer treatment plan, including pedal and nail manifestations, along with presenting relevant cases where podiatric intervention had an impact.
Targeted cancer therapies and chemotherapy can result in a wide range of dermatologic adverse events. Chemotherapy consists of drugs that effect cells that are undergoing mitosis versus targeted therapy which are drugs that inhibit a more specific target in cells. Toxicities of these drugs include damage to the skin, oral mucosa, hair, and nails in nearly all patients.1 An estimated 17 million individuals received a cancer diagnosis worldwide in 2018, many of these individuals will or have received systemic anticancer therapy.2 By 2040, the global burden is expected to grow to 27.5 million new cancer cases due to population growth and aging.2 Dermatologic side effects (including those in the lower extremity) can significantly impact a patient’s quality of life. Managing these toxicities are important for for overall improvement of cancer patient outcomes. Frequently the treatment team may find it necessary to modify the dose of therapy based on dermatologic adverse events. Therefore, an interdisciplinary collective effort is essential to comprehensively care for patients who receive anti-cancer treatment.
Understanding Pertinent Dermatological Manifestations Of Cancer Treatment
The impact of these toxicities is generally more severe with targeted therapy than with chemotherapy.3 Rash is the most common skin reaction and is typically the earliest adverse event.4 Acneiform rash develops in the majority of patients treated with inhibitors of epidermal growth factor receptor inhibitor (EGFRI) and mitogen-activated protein (MAP) and kinase kinase inhibitor (MEKI).3 In a study by Braden and colleagues,5 which involved 157 patients with EGRFI-induced skin reactions, papulopustural eruptions (acneiform rash) occurred on average at 9.4 weeks and mostly involved the face (97 percent), chest (75 percent) and back (61 percent). Involvement of the lower extremity was less frequently observed at four percent.5 This rash can occur within two to four weeks of treatment, characterized by papules and pustules, itching, severe pain and spontaneous bleeding of lesions.3
Hand-Foot Syndrome (HFS), also known as palmar-plantar erythrodysesthesia or acral erythema, is a well-documented adverse effect of many chemotherapeutic agents.6 Pegylated liposomal doxorubicin, capecitabine, 5-fluorouracil, cytarabine, and docetaxel most commonly cause this condition. Newer targeted multikinase inhibitors (MKIs) such as sorafenib, sunitinib, axitinib, pazopanib, regorafenib and vemurafenib can also cause a reaction in the hands and feet.6 The incidence rate varies from six to 64 percent and also varies with causative agent.6 HFS incidence is about 30 percent with vemurafenib or dabrafenib. When combined with an MEKI the incidence decreases to six to 10 percent. Certain chemotherapeutic drug combinations can increase the risk of HFS, for instance, doxorubicin with continuous 5-fluorouracil (5-FU) has a reported incidence of HFS of 89 percent. The risk of getting HFS is dose-dependent for all treatments.6
HFS has an onset within two to twenty-one days but can present up to ten months after treatment. This can begin with a tingling sensation, which progresses to burning pain within several days, believed to be caused by small fiber neuropathy.7 A plaque of palmar-plantar erythema and edema accompanies neuropathic symptoms. The erythema can progress to blistering with subsequent erosion and ulceration. HFS can present with hyperpigmentation in the African-American population.7 HFS is more common in the palms than the soles and can involve the dorsal hands and feet, mostly prominent on the lateral aspects of the fingers and distal fat pads. HFS can severely impact a patient’s ability to walk. Treatment of HFS includes the use of keratolytic agents and gentle debridement.7 Hyperkeratotic lesions can occur with a v-raf murine sarcoma viral oncogene homolog B1 inhibitor (BRAFI) when used as monotherapy, including verrucous-type keratosis or a keratosis pilaris rash (more common on upper outer arms but can occur on dorsal feet, but not the soles).7
Chemotherapy with BRAFIs, used for advanced metastatic melanoma, is associated with a 20 percent incidence of secondary skin tumors such as squamous cell carcinoma and keratoacanthomas.8 There is also documentation of a maculopapular hypersensitivity-like rash in patients with advanced melanoma treated with vemurafenib who had prior immunotherapy with ipilimumab or nivolumab.1 The rash is thought to be a non-specific inflammatory reaction. When it is not possible to switch therapy when one first notices a hypersensitivity reaction, an alternative option may be to discontinue therapy and prescribe oral corticosteroids until symptoms resolve. With resolution of symptoms, restarting treatment will not always create the same reaction.1
What You Should Know About Nail Changes With Anti-Cancer Therapy
Chemotherapy-induced nail toxicity can result in cosmetic concerns, pain and infection. Onycholysis can occur with taxanes such as paclitaxel and more frequently, docetaxel. Incidence ranges from zero to 44 percent. Additional taxane-related nail changes include Beau’s lines, subungual hemorrhage, nail pigmentation, acute paronychia and splinter hemorrhage.9
Targeted therapies can cause damage to the nail folds, such as paronychia and periungual pyogenic granulomas.9 Treatment for pyogenic granuloma is a partial nail avulsion with destruction of the granulation tissue and possibly phenolization.9 It is important to educate patients on preventative strategies to avoid adverse effects of targeted therapies. Patients should avoid repeated trauma or friction to the nails and the nail beds. They should use protective gloves, avoid prolonged contact with water and should not use nail polish removers and hardeners. Patients should trim their nails regularly and wear wide, comfortable footwear with cotton socks. This should all be initiated upon beginning of treatment with targeted therapy.9
Additionally, anthracyclines such as doxorubicin, daunorubicin and idaubicin, can cause both diffuse and banded (longitudinal and transverse) patterns of nail pigmentation. This can resolve with discontinuation of therapy and nail growth.9
When Patients Present With Pedal Dermal Events From Anti-Cancer Therapy
Case #1. A 72-year-old male with a past medical history of coronary artery disease, poorly-controlled type 2 diabetes mellitus, hypertension, hyperlipidemia and colorectal cancer presented to the office on referral from the emergency department due to severe pain with ambulation as well as a two-week history of pedal blistering and erosions. He had undergone colon resection six months prior, preceded by chemotherapy and radiation. He continued to take capecitabine (Xeloda®, Genentech) postoperatively for his cancer. At the time of his visit there was no evidence of metastasis.
On exam the patient exhibited bilateral plantar exfoliation and xerosis with fissuring. Superficial erosions were evident at the plantar hallux bilaterally, plantar-medial right first metatarsophalangeal joint and the distal tips of digits two, three, four, and five on the right foot. We also observed onycholysis to all nails of the right foot and the first and fifth nails on the left foot.

We arrived upon a diagnosis of exfoliative dermatitis and onycholysis secondary to his chemotherapy. After consultation with his oncologist, the team advised the patient to discontinue the capecitabine. We also prescribed ammonium lactate 12% lotion and instructed the patient to use topical antibiotic ointment daily to the eroded areas.
He presented the office for follow-up three weeks after discontinuation of his medication, stating he was now able to walk. His exam showed almost 100 percent resolution of the exfoliation and xerosis with fissuring of his plantar feet. The superficial erosions of his plantar hallux bilaterally were healed, along with the lesions of the distal tips of digits two, three, four, and five of his right foot. Onycholysis had also resolved . We instructed the patient to continue to use ammonium lactate lotion 12% daily to the feet after showering.

Case #2. A 39-year-old female presented with a chief concern of onycholysis of the hallux nails with a subungual infection of the left hallux. The patient was undergoing treatment for breast cancer with a double mastectomy planned within the next few days of her initial visit. She was on paclitaxel (Taxol) chemotherapy. The patient had no other significant medical history. On exam, the hallux nails bilaterally presented with total lysis, subungual serosanguinous fluid and multiple pyogenic granulomas in the nail beds.
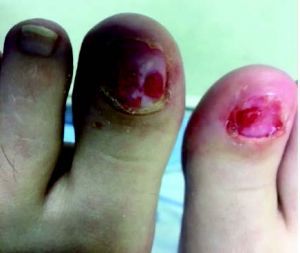
We diagnosed this patient with pyogenic granulomas secondary to paclitaxel chemotherapy. Treatment consisted of careful aseptic debridement of all lysed portions of the toenails to expose granulation tissue. We then cauterized the granulation tissue with silver nitrate and applied a sterile dressing with topical bacitracin ointment. The patient received instructions to wash the area daily with soap and water and apply a topical antibiotic dressing.
A week later the patient followed up stating she felt much better. At this time, her pyogenic granulomas showed signs of resolution and we discontinued the antibiotic dressing. In discussion with the patient, we addressed the potential issues with nail regrowth, including continued lysis and paronychia. Approximately two months later the patient followed up again with full resolution of her pyogenic granulomas of the bilateral great toes, but she exhibited continued nail dystrophy. We debrided the lysed portions of the nail plate and reinforced the discussion of the potential future dermatologic issues secondary to her paclitaxel chemotherapy.
In Conclusion
Collaboration between podiatrists and oncologists is crucial for the successful treatment of skin and nail toxicities attendant to systemic oncologic therapies. These cases above demonstrate two situations, one where the patient had complete resolution of symptoms after discontinuation of therapy. His quality of life greatly improved and he regained the ability to walk without pain. The second patient was unable to stop her therapy. It is important in these situations to treat the pathology with supportive therapy and educate the patient on any possibility of reoccurrence of symptoms.
Dermatologic toxicities related to targeted cancer therapies and chemotherapy can also contribute to psychosocial issues, and thus can severely diminish a patient’s quality of life at a very vulnerable time. This may impact adherence to their anti-cancer treatment and thus jeopardizes its success. It is important to identify and appropriately treat these adverse events as part of the the comprehensive treatment team.
Dr. Kaufman is the Chief Podiatric Medicine and Surgery Resident at Mount Sinai Medical Center in New York.
Dr. Markinson is an Associate Professor in the Department of Orthopedic Surgery, an Instructor in the Department of Dermatologic Surgery and a Team Member of the Melanoma and Skin Cancer Program at the Tisch Cancer Institute of the Icahn School of Medicine at Mount Sinai Medical Center in New York.
1. Lacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 2018;19(S1):31–39.
2. American Cancer Society. Global cancer facts and figures. Available at: https://www.cancer.org/ research/cancer-facts-statistics/global.html . Accessed April 7, 2021.
3. Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803-812.
4. Peng Y, Li Q, Zhang J, et al. Update review of skin adverse events during treatment of lung cancer and colorectal carcinoma with epidermal growth receptor factor inhibitors. BioSci Trends. 2018;12(6):537–552.
5. Braden RL, Anadkat MJ. EGFR inhibitor-induced skin reactions: Differentiating acneiform rash from superimposed bacterial infections. Support Care Cancer. 2016; 24:3943-3950.
6. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71(4):787-794.
7. Stubblefield MD, Custodio CM, Kaufmann P, Dickler MN. Small-fiber neuropathy associated with capecitabine (Xeloda)-induced hand-foot syndrome: a case report. J Clin Neuromuscul Dis. 2006;7:128-132.
8. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibiton in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694-1703.
9. Piraccini BM, Bellavista S, Misciali C, Tosti A, de Berker D, Richert B. Periungual and subungual poygenic granuloma. Br J Dermatol. 2010;163(5);941-953.
Additional References
10. Belum VR, Marulanda K, Ensslin C, et al. Alopecia in patients treated with molecularly targeted anticancer therapies. Ann Oncol. 2015;26(12):2496- 2502.
11. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012; 367(2):107-114.
12.. Perier-Muzet M, Thomas L, Poulalhon N, et al. Melanoma patients under venurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. J Invest Dermatol. 2014;134(5);1351- 1358.
13. Piraccini BM, Patrizi A, Fanti PA, et al. RASpoathic alopecia: hair changes associated with vemurafenib therapy. J Am Acad Dermatol. 2015;72(4):734-741.
14. Saggar V, Wu S, Dickler MN, Lacouturue ME. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18(10):1126-1134.
15. Robert C, Sibaud V, Mateus C, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16(4):941-953
16. Virgarios E, Epstein JB, Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer. 2017;25(5):1713-1739.
17. von Gruenigen V, Frasure H, Fusco N, et al. A double-blind, randomized trial of pyridoxine versus placebo for the prevention of pegylated liposomal doxorubicin-related hand-foot syndrome in gynecologic oncology patients. Cancer. 2010;116:4735-4743.











