Essential Pearls On Skin Grafting
Recent literature has suggested that split-thickness skin grafts (STSGs) have a growing role in wounds, particularly in the diabetic foot. These authors provide practical pearls for preparing the STSG application site, harvesting the graft and applying the graft successfully.
Skin grafting is a technique that dates back thousands of years.1 The progression of its utility through time has advanced from limited application on noses to extremities and presently to the impressive surgical coverage of large soft tissue defects.1,2
 Thiersch first described the use of split-thickness skin grafts (STSG) in 1939.3 The eventual invention of a calibrated dermatome increased the popularity and harvesting process of STSG as it allowed for a free hand during surgery, and facilitated a reproducible and predictable procedure.4
Thiersch first described the use of split-thickness skin grafts (STSG) in 1939.3 The eventual invention of a calibrated dermatome increased the popularity and harvesting process of STSG as it allowed for a free hand during surgery, and facilitated a reproducible and predictable procedure.4
Recently, physicians have increasingly utilized STSG and reported it to be safe and effective in the coverage of diabetic foot ulcerations (DFUs).5-9 There is a general historic paucity of literature concerning the application of the STSG in the treatment of diabetic foot ulcers. More recent literature, however, reveals successful application of healing DFUs with the application of a STSG.
Santema and colleagues performed a Cochrane Database systematic review that assessed 17 randomized studies that involved a total of 1,655 participants with a DFU who received either a skin substitute or standard wound care.5 The authors reported increased healing rates and a decreased rate of amputation with the use of a skin substitute for the treatment of DFUs versus standard of care.
An additional report by Mahmoud and coworkers comparing STSG versus non-surgical therapy for DFU found that 86 percent of patients healed at eight weeks in the STSG group versus 46 percent of patients treated with a conservative dressing.6
In a review of 83 patients with DFUs who had STSG placement, Ramanujam and colleagues reported that 65 percent of patients healed within seven weeks postoperatively with uneventful progression to wound closure.7
A further retrospective examination of 107 patients with non-healing diabetic foot or leg ulcers who had STSG application reported a mean time to healing of 5.1 weeks.8 Researchers also reported a low complication incidence of 2.8 percent.
Rose and colleagues performed a five-year retrospective review of 94 patients with chronic lower limb wounds, 66 of whom had diabetes and 13 of whom were on dialysis.9 They reported that greater than 65 percent of patients had complete graft incorporation and healing, 18 percent required revision, and five patients ultimately required major limb amputation. The researchers cited statistically significant differences in the healing of wounds in patients with diabetes in comparison to patients without diabetes, and in comparing plantar versus non-plantar graft application sites. Patients with dialysis had higher rates of revision but there was no difference in wound healing as a function of time when comparing these patients to those not on dialysis.
Preparing The Site For Rapid Skin Grafting
The harvested portion of skin for STSG includes the entire epidermal layer with varying portions of dermis depending on the depth setting of the dermatome. The thickness of STSG can be thin (0.2 to 0.3 mm), medium (0.4 to 0.5 mm) or thick (0.6 to 0.7 mm) respectively. We prefer a setting of “16-18” on the dermatome, which is equivalent to 0.016 to 0.018 inches (or 0.40 to 0.45 mm). When it comes to the donor site, there are multiple areas to consider including the buttocks, the scalp in young patients, any area of the calf and the thigh, which is our donor site of choice. In evaluating the body surface for appropriate donor sites, considerations include searching for locations that are minimally conspicuous and devoid of frequent or recurrent pressure, the latter of which one should use in an effort to reduce pain associated with the donor site.
Surgeons should ensure the STSG can sufficiently fill the vertical defect with coverage of vital structures and healthy covering of well-perfused, healthy granular tissue. Preoperatively, one should also be aware of the potential complications, cost and time constraints with STSG application. One would reserve STSG application for appropriate wounds of sufficient size and delayed healing that would indicate a need for advanced wound healing procedures. Very small wounds often heal with non-surgical or advanced biological therapies so STSG application would not be indicated in these clinical scenarios.
Intraoperative wound preparation includes the removal of all superficial, exudative films, detritus and areas of necrosis. We prefer hydrodebridement such as with Versajet (Smith & Nephew) and dermal curettage for intraoperative wound preparation. Other historic yet highly effective debridement instruments include the Weck blade, also known as a Goulian skin grafting knife. This instrument has historic use in harvesting graft tissue but with modern dermatomes in use for graft harvest, the Weck blade is proving useful in the stages of wound bed preparation. Upon completion of surgical wound preparation, the final step before graft harvesting procedures includes washing the wound with the bristle side of a surgical hand-washing sponge and rinsing with pulse lavage.
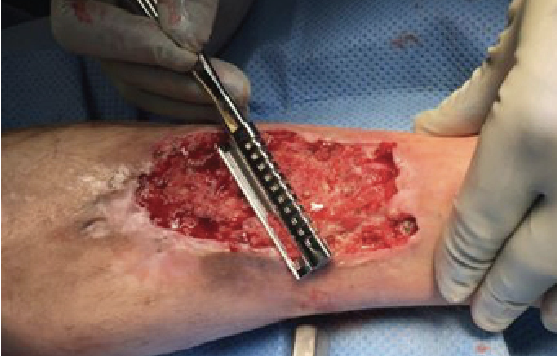
 In preparation for graft harvesting, measure the wound. Subsequent evaluation and marking of the donor site should reflect the anticipated graft size to cover the defect. After appropriately marking the site, infiltrate the donor site superficially with 1% lidocaine and epinephrine or with 0.25% bupivacaine and epinephrine to create a uniform wheal of the donor tissue and promote hemostasis. Using the dermatome blade guide as a width gauge before securing the blade to the dermatome is often beneficial in determining the appropriate guide (graft) width. Confirm your preference of dermatome settings including the appropriate width and depth. Also evaluate blade/guide clearance. To do this, insert a surgical blade between the blade and the blade guide to confirm patency and uniformity. The application of mineral oil to the blade, blade guide and the donor site allow for smooth advancement during the harvesting process.
In preparation for graft harvesting, measure the wound. Subsequent evaluation and marking of the donor site should reflect the anticipated graft size to cover the defect. After appropriately marking the site, infiltrate the donor site superficially with 1% lidocaine and epinephrine or with 0.25% bupivacaine and epinephrine to create a uniform wheal of the donor tissue and promote hemostasis. Using the dermatome blade guide as a width gauge before securing the blade to the dermatome is often beneficial in determining the appropriate guide (graft) width. Confirm your preference of dermatome settings including the appropriate width and depth. Also evaluate blade/guide clearance. To do this, insert a surgical blade between the blade and the blade guide to confirm patency and uniformity. The application of mineral oil to the blade, blade guide and the donor site allow for smooth advancement during the harvesting process.
How To Harvest The Graft And Perform Skin Meshing
Harvest the graft by applying the activated dermatome to the donor site. Maintaining the dermatome at a 45-degree angle to the skin facilitates sufficient blade purchase and graft lift from donor tissues. Applying moderate pressure is key as too little pressure will produce non-uniform and often laced grafts. Too much pressure may produce irregular advancement and often, paradoxically, may produce graft lacing as well. Surgeons should also ensure smooth advancement, which allows for the atraumatic removal of graft tissue and prevents tearing of the harvested graft.
When possible, have an assistant lift the graft from the face of the blade as you advance the dermatome. This prevents bunching on the blade face. Upon reaching the donor site terminus, lift the dermatome in a smooth, upward sweeping motion from the donor site until it is completely free from the surface of the skin. Then apply the graft to the appropriate meshing plate and transfer the graft to the back table. One can apply saline to the graft and meshing plate to facilitate the proper expansion of the graft during the meshing process. The surgeon may then dress the donor site according to preference. We prefer petrolatum gauze directly over the donor site and securing it with fenestrated Tegaderm (3M), which the surgeon can suture in place with 4-0 chromic gut suture to reduce dressing movement and increase patient comfort. Often, we will apply autologous platelet rich plasma to the donor site, which research has shown to reduce postoperative pain.10 Wrap all of this with appropriate gauze coverage and Army battle dressings, and secure it with cast padding and ACE bandages.
Perform graft meshing as per your preference. The surgeons can use the mesher to perforate the sheet graft mechanically to increase coverage surface area and provide an avenue for wound exudates and sanguineous fluid from the wound-graft interface. Facilitating the removal of serous and blood products reduces hematoma and seroma formation, which inhibit graft adherence.11,12 Meshing also increases plasticity, promotes angiogenesis and provides consistent results during grafting procedures on the diabetic foot.11-13 One may also perform pie crusting, the manual perforating of a graft with sharp instrumentation, at this time for the same purpose.
Key Pointers On Securing And Dressing The STSG
The surgeon transfers the STSG to the recipient site and secures it in place utilizing various means. We prefer 4-0 chromic gut suture or surgical staples. Each has its benefits and drawbacks. Chromic gut sutures promote an inflammatory response and edge purchase whereas staples do not. However, one must remove staples manually after surgery whereas the body absorbs chromic gut sutures.
In dressing the newly applied skin graft, traditional saline moist bolster dressings have fallen out of favor with negative pressure wound therapy (NPWT) being increasingly common. Negative pressure increases graft take with a decreasing occurrence of hematoma and seroma postoperatively.14,15 The NPWT system can remain intact for five to seven days postoperatively with reapplication as appropriate per physician preference and follow-up findings.
The postoperative course varies depending on each evaluation. Physicians must be prepared to alter the course of care when presented with maceration, infection and colonization, or other findings. High quality wound evaluation and care remain the basis for effective postoperative graft treatment.
Upon wound healing, one should routinely moisten the STSG as the dermal appendages of the donor site do not transfer with the graft and the recipient site is inherently devoid of these same structures. Patients should wear custom offloading trilaminar inserts and diabetic shoes when appropriate. Often one must make a new casting for inserts after the healing of a STSG. Follow-up care should be based on the American Diabetes Association guidelines or physician preference.16
 Pertinent Procedure Tips And Pearls
Pertinent Procedure Tips And Pearls
Thoroughly evaluate and select each patient for the application of STSG when medically appropriate. Ramanujam and coworkers conducted a retrospective review of approximately 200 patients with diabetes and foot wounds who had subsequent STSG placement.7 The authors found that the comorbidities associated with diabetes, such as peripheral vascular disease, retinopathy, nephropathy and cardiovascular disease, were a greater indication of graft failure than a sole diagnosis of diabetes. Working in concert with other members of the multidisciplinary team, one should assess and help the patient optimize his or her vascular, renal, glycemic and overall systemic state to increase the chance of skin graft incorporation.
Preoperative wound preparation should lead to an ulceration that is clear of infection, has minimal depth and a granular wound base that covers all areas of deep tissue including tendons, ligaments and bones. A review of systems should reveal any other areas of skin breakdown and possible additional sites of infection. To reduce the risk of introducing new infection to the carefully prepared STSG site, all patients will ideally be free of other areas of soft tissue infection at the time of the procedure. One should allow recipient site wounds sufficient time for adequate filling and coverage. Defer the application of STSG until the wound reaches this vital state.
The electric dermatome is a remarkable advancement in the harvesting of STSG. However, one must understand and review its use prior to each harvesting procedure. As we mentioned above, the physician should apply it with moderate pressure, advance the dermatome smoothly, maintain the dermatome at a 45-degree angle, have an assistant lift the graft and remove the dermatome with a smooth upstroke.
Consider patient appropriate dressings during surgical planning. Hypothetically, social or financial constraints may prohibit the use of NPWT after a graft application. If one is considering NPWT, however, order a portable NPWT machine in advance to be delivered to the patient’s home. This simple step can easily prevent delays on the day of surgery due to insufficient or inappropriate supplies. We routinely apply NPWT for at least two weeks prior to the application of STSG. This can provide healthy and clean tissue on the day of operation.
Use V.A.C.® WhiteFoam Dressings (Kinetic Concepts Incorporated) for direct contact with the STSG. This foam is a specially formulated polyvinyl alcohol substance, which is designed to be less adherent for the specific use of STSG. One then covers this layer with regular black foam (V.A.C.® GranuFoam™, KCI) and secures the layer in the typical NPWT fashion.
Use surgical staples to secure the STSG into place. This reduces intraoperative time, mitigates the risks of inadvertent needle sticks and reduces soft tissue reaction in comparison to other means of securing STSG.
Protect the graft recipient site from shear and compressive forces. The interface between the new graft and the recipient site remains delicate, and is subject to inadvertent forces for several weeks. Postoperative protective measures include the use of splinting and padding. Immediately after surgery, provide copious layers of cast padding and also make an effort to decrease movement of the dressings due to NPWT hose movement. Often, one may apply a posterior splint to maintain rectus foot positioning and decrease the risk of inadvertent trauma to the surgical site.
Be vigilant in evaluation for Pseudomonas colonization. Split-thickness skin grafts in particular are remarkably vulnerable to devastating destruction due to P. aeruginosa colonization.17,18 Colonization is usually evident by the indescribable yet immediately recognizable odor of Pseudomonas. Colonization may or may not be evident by the scant or sometimes florid, verdant hue with maceration of STSG. If one suspects pseudomonal colonization, acetic acid and Dakin’s moist bolster-type dressings have proven to be effective in controlling the bacterial course.17,18
Finally, be patient. Each wound’s consistency, makeup, physiology and even physical shape alters the course and outcome of STSG “take” and progression. It is our experience that STSGs advance through a post-op healing evolution, which often includes evaluations that reveal questionable graft success. Continuing with the routine postoperative course and allowing subtle changes to promote high quality healing as each case requires frequently leads to successful graft application and eventual wound healing.
In Conclusion
The use of split thickness skin graft is a powerful method in the healing of healthy yet chronic diabetic foot wounds. Surgeons can easily learn and reproduce the STSG procedure. The procedure is effective in the coverage of deficits of many shapes and sizes. We have provided the aforementioned tips and pearls as considerations during the process of each STSG procedure. Before the procedure, however, a surgeon’s comprehensive understanding of preoperative indications, intraoperative procedure and postoperative course will prove to be the most critical of all instruments in this process.
Dr. Hatch is a second-year resident within the Tucson Medical Center/Midwestern University residency program in podiatric medicine and surgery.
Dr. Armstrong is the Director of the Southern Arizona Limb Salvage Alliance and a Professor of Surgery at the University of Arizona Medical Center in Tucson, Ariz.
References
- Hauben DJ, Baruchin A, Dan M. On the history of the free skin graft. Ann Plast Surg. 1982; 9(3):242–246.
- Reverdin JL, Ivy RH. The classic reprint greffe epidermique—experience faite dans le service de M. le Docteur Guyon, a Lʼhôpital Necker. Plast Reconstr Surg. 1968; 41(1):79–81.
- Padgett EC. Skin grafting in severe burns. Am J Surg. 1993; 43(2):626–636.
- Ketchum LD. An historical account of the development of the calibrated dermatome. Ann Plast Surg. 1978; 1(6):608–611.
- Santema TB, Poyck PPC, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016; 2: CD011255.
- Mahmoud SM, Mohamed AA, Mahdi SEI, et al. Split-skin graft in the management of diabetic foot ulcers. J Wound Care. 2008; 17(7):303–306.
- Ramanujam CL, Stapleton JJ, Kilpadi KL, et al. Split-thickness skin grafts for closure of diabetic foot and ankle wounds: a retrospective review of 83 patients. Foot Ankle Spec. 2010; 3(5):231–240.
- Anderson JJ, Wallin KJ, Spencer L. Split thickness skin grafts for the treatment of non-healing foot and leg ulcers in patients with diabetes: a retrospective review. Diabet Foot Ankle. 2012; epub Feb. 20.
- Rose JF, Giovinco N, Mills JL, et al. Split-thickness skin grafting the high-risk diabetic foot. J Vasc Surg. 2014; 59(6):1657–1663.
- Miller JD, Rankin TM, Hua NT, et al. Reduction of pain via platelet-rich plasma in split-thickness skin graft donor sites: a series of matched pairs. Diabet Foot Ankle. 2015; 6: 24972.
- Glogau RG, Stegman SJ, Tromovitch TA. Refinements in split-thickness skin grafting technique. J Dermatol Surg Oncol. 1987; 13(8):853–859.
- Knight SL, Moorghen M. Configurational changes within the dermis of meshed split skin grafts: a histological study. Br J Plast Surg. 1987; 40(4):420–422.
- Puttirutvong P. Meshed skin graft versus split thickness skin graft in diabetic ulcer coverage. J Med Assoc Thai. 2004; 87(1):66–72.
- Blackburn JH 2nd, Boemi L, Hall WW, et al. Negative-pressure dressings as a bolster for skin grafts. Ann Plast Surg. 1998; 40(5):453–457.
- Isaac AL, Rose J, Armstrong DG. Mechanically powered negative pressure wound therapy as a bolster for skin grafting. Plast Reconstr Surg. 2014; 2(2):e103.
- Armstrong DG, Abu-Rumman PL, Nixon BP, et al. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001; 91(9):451–455.
- Phillips I, Lobo AZ, Fernandes R, et al. Acetic acid in the treatment of superficial wounds infected by Pseudomonas aeruginosa. Lancet. 1968; 1(7532):11–14.
- Sloss JM, Cumberland N, Milner SM. Acetic acid used for the elimination of Pseudomonas aeruginosa from burn and soft tissue wounds. J Royal Army Medical Corps. 1993; 139(2):49–51.











