Emerging Concepts In The Etiology Of Charcot Joints
July 2014
With new developments in the research on Charcot neuroarthropathy, these authors examine emerging research findings on osteoclastic activity, the RAGE pathway and cytokine recruitment, and share insights on current imaging tools and treatment modalities.
 While the treatment modalities and physiological understanding of Charcot neuroarthropathy may be much different today than in 1827, the presentation has remained relatively the same.1 Classically, Charcot neuroarthropathy presents as a unilateral redness and swelling of a lower extremity. Typically, this increase in warmth precipitates bony changes visible on X-rays and bone scans.
Due to coexisting peripheral neuropathy, only around 30 percent of patients will notice pain or discomfort prior to these changes.2 In our experience, acute trauma is often a common trigger for a Charcot “flare-up.” However, researchers report this in only 22 to 53 percent of patients.3 Currently, Charcot neuroarthropathy is one of the most devastating conditions secondary to diabetes, affecting up to 13 percent of high-risk patients with diabetes mellitus.4
Modern treatment modalities for Charcot foot center on immobilization and offloading either by total contact casts or removable controlled ankle motion (CAM) boot walkers.5 These attempts at preventing further deformity minimize the chance of ulceration leading to amputation. Surgical debridement and episodic reconstructions are also common, despite their high cost and unlikeliness to resolve the causative underlying physiologic mechanisms. These limitations typically keep these treatments from being functionally first-line treatment efforts.
We commonly prescribe adjunctive therapies such as biguanides and bisphosphonates in the acute Charcot osteoarthropathy although current evidence is inconclusive.6,7 Larger clinical trials are still needed to establish the efficacy of pharmaceutical intervention.
While the treatment modalities and physiological understanding of Charcot neuroarthropathy may be much different today than in 1827, the presentation has remained relatively the same.1 Classically, Charcot neuroarthropathy presents as a unilateral redness and swelling of a lower extremity. Typically, this increase in warmth precipitates bony changes visible on X-rays and bone scans.
Due to coexisting peripheral neuropathy, only around 30 percent of patients will notice pain or discomfort prior to these changes.2 In our experience, acute trauma is often a common trigger for a Charcot “flare-up.” However, researchers report this in only 22 to 53 percent of patients.3 Currently, Charcot neuroarthropathy is one of the most devastating conditions secondary to diabetes, affecting up to 13 percent of high-risk patients with diabetes mellitus.4
Modern treatment modalities for Charcot foot center on immobilization and offloading either by total contact casts or removable controlled ankle motion (CAM) boot walkers.5 These attempts at preventing further deformity minimize the chance of ulceration leading to amputation. Surgical debridement and episodic reconstructions are also common, despite their high cost and unlikeliness to resolve the causative underlying physiologic mechanisms. These limitations typically keep these treatments from being functionally first-line treatment efforts.
We commonly prescribe adjunctive therapies such as biguanides and bisphosphonates in the acute Charcot osteoarthropathy although current evidence is inconclusive.6,7 Larger clinical trials are still needed to establish the efficacy of pharmaceutical intervention.
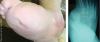 There have been two main competing theories behind the etiology of Charcot development. Classically, the neurovascular theory holds that vascular damage to the autonomic nervous system causes a reflex hyperemia and an increase in blood flow to the limbs via arteriovenous shunts.7 The second theory holds that mechanical microtrauma initiates an inflammatory cascade that self-perpetuates into gross bony breakdown.7 However, it is more likely that both of these theories function together as autonomic dysfunction inhibits the perception of repetitive stress amid the accumulation of circulating inflammatory cytokines.
However, neither theory explains why Charcot episodes typically develop unilaterally and occur in only a small percentage of patients with diabetic neuropathy. Also, Charcot can develop secondary to vascular disease, skirting many of the aforementioned pathways.8 Foot and ankle specialists often relate the incidence of inflammation (itself a possible initiator of Charcot episodes) with gross bony changes. At times, this may appear secondary to infection and can be misleading to unfamiliar diagnosticians, such as clinicians at urgent care facilities.
While the mechanisms connecting Charcot’s inflammatory cascade to its phenotypic disease state presentation are wholly unknown, new research in the physiological changes that occur during neuroarthropathic breakdown is unveiling a more quantified disease mechanism. This emerging research could lead to better means of early diagnosis as well as improved medical management.
There have been two main competing theories behind the etiology of Charcot development. Classically, the neurovascular theory holds that vascular damage to the autonomic nervous system causes a reflex hyperemia and an increase in blood flow to the limbs via arteriovenous shunts.7 The second theory holds that mechanical microtrauma initiates an inflammatory cascade that self-perpetuates into gross bony breakdown.7 However, it is more likely that both of these theories function together as autonomic dysfunction inhibits the perception of repetitive stress amid the accumulation of circulating inflammatory cytokines.
However, neither theory explains why Charcot episodes typically develop unilaterally and occur in only a small percentage of patients with diabetic neuropathy. Also, Charcot can develop secondary to vascular disease, skirting many of the aforementioned pathways.8 Foot and ankle specialists often relate the incidence of inflammation (itself a possible initiator of Charcot episodes) with gross bony changes. At times, this may appear secondary to infection and can be misleading to unfamiliar diagnosticians, such as clinicians at urgent care facilities.
While the mechanisms connecting Charcot’s inflammatory cascade to its phenotypic disease state presentation are wholly unknown, new research in the physiological changes that occur during neuroarthropathic breakdown is unveiling a more quantified disease mechanism. This emerging research could lead to better means of early diagnosis as well as improved medical management.
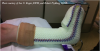 Osteoblasts can also inhibit the differentiation of osteoclasts by secreting osteoprotegerin, a decoy competitor ligand to RANKL.12 Osteoprotegerin acts as a competitive inhibitor to the RANKL by binding to the RANK surface membrane. Animal studies have demonstrated this to reduce the differentiation and production of osteoclast cells.9,13 Further study on this topic is currently underway and the effects may help reverse or reduce the symptoms of diseases such as osteopenia, osteoporosis, Charcot foot and other bony dysfunctions.
Calcitonin also has an important role in the differentiation of osteoclasts. Calcitonin, released by C cells in the thyroid, has a physiologic role in the body in decreasing serum calcium levels in the body. Calcitonin may also directly inhibit osteoclastic differentiation and activity via calcitonin receptors on osteoclasts.14,15
Osteoblasts can also inhibit the differentiation of osteoclasts by secreting osteoprotegerin, a decoy competitor ligand to RANKL.12 Osteoprotegerin acts as a competitive inhibitor to the RANKL by binding to the RANK surface membrane. Animal studies have demonstrated this to reduce the differentiation and production of osteoclast cells.9,13 Further study on this topic is currently underway and the effects may help reverse or reduce the symptoms of diseases such as osteopenia, osteoporosis, Charcot foot and other bony dysfunctions.
Calcitonin also has an important role in the differentiation of osteoclasts. Calcitonin, released by C cells in the thyroid, has a physiologic role in the body in decreasing serum calcium levels in the body. Calcitonin may also directly inhibit osteoclastic differentiation and activity via calcitonin receptors on osteoclasts.14,15
 However, proper surveillance and early detection are often aberrant. A majority of patients with acute Charcot episodes do not receive treatment or, in many cases, are altogether misdiagnosed. Preventative and therapeutic medical management options are sparse and widespread efforts to increase patient and provider education are still warranted.
Previously, bisphosphonates were among the few drugs known to help acute Charcot patients. However, recent studies validated the use of intranasal calcitonin as an effective alternative for acute Charcot.3,4 More importantly, studies have shown calcitonin to be safe in patients with renal insufficiency, a complication limiting bisphosphonate use.3,4
In 2006, Bem and colleagues showed the efficacy of intranasal calcitonin for decreasing the markers of bone turnover in acute Charcot neuroosteoarthropathy.14 In contrast to bisphosphonates that decrease bone resorption as well as bone formation markers, calcitonin does not decrease bone formation markers. As we mentioned above, osteoclasts have specific calcitonin receptors that bind calcitonin. By directly inhibiting osteoclasts, interventional calcitonin may effectively decrease bone resorption without altering osteoblastic activity.
The RANK ligand inhibitors are human monoclonal antibodies that have affinity and specificity to RANK ligand. Recently, an eight-year study of a phase 2 clinical trial showed denosumab to be a potent inhibitor of bone turnover in postmenopausal women.20 McClung and coworkers showed significant increases in patients’ bone marrow densities and reductions on bone tumor markers.20
Currently, RANKL inhibitors such as denosumab only have FDA approval for the following:
• unresectable giant cell tumors of bone in adults and skeletally mature adolescents;
• increasing bone mass in patients at high risk for fracture including those taking androgen deprivation therapy for nonmetastatic prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer;
• prevention of skeletal-related events in patients with bone metastases from solid tumors; and
• treatment of postmenopausal women with osteoporosis at high risk for fracture.21,22
Soluble RAGE mimic is another avenue that is currently under investigation for medical management of Charcot neuroarthropathy. Witzke and colleagues demonstrated that patients with diabetes without Charcot neuroarthropathy had reduced serum levels of sRAGE by 50 percent and those with Charcot neuroarthropathy had 87 percent lower serum levels of sRAGE in comparison to the healthy control group.23 Current investigations are aiming to guage the efficacy of sRAGE ligand decoys. Ramasamy and colleagues deduce that RAGE inhibition would not carry physiological adverse effects or suppress the innate immune system.24
However, proper surveillance and early detection are often aberrant. A majority of patients with acute Charcot episodes do not receive treatment or, in many cases, are altogether misdiagnosed. Preventative and therapeutic medical management options are sparse and widespread efforts to increase patient and provider education are still warranted.
Previously, bisphosphonates were among the few drugs known to help acute Charcot patients. However, recent studies validated the use of intranasal calcitonin as an effective alternative for acute Charcot.3,4 More importantly, studies have shown calcitonin to be safe in patients with renal insufficiency, a complication limiting bisphosphonate use.3,4
In 2006, Bem and colleagues showed the efficacy of intranasal calcitonin for decreasing the markers of bone turnover in acute Charcot neuroosteoarthropathy.14 In contrast to bisphosphonates that decrease bone resorption as well as bone formation markers, calcitonin does not decrease bone formation markers. As we mentioned above, osteoclasts have specific calcitonin receptors that bind calcitonin. By directly inhibiting osteoclasts, interventional calcitonin may effectively decrease bone resorption without altering osteoblastic activity.
The RANK ligand inhibitors are human monoclonal antibodies that have affinity and specificity to RANK ligand. Recently, an eight-year study of a phase 2 clinical trial showed denosumab to be a potent inhibitor of bone turnover in postmenopausal women.20 McClung and coworkers showed significant increases in patients’ bone marrow densities and reductions on bone tumor markers.20
Currently, RANKL inhibitors such as denosumab only have FDA approval for the following:
• unresectable giant cell tumors of bone in adults and skeletally mature adolescents;
• increasing bone mass in patients at high risk for fracture including those taking androgen deprivation therapy for nonmetastatic prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer;
• prevention of skeletal-related events in patients with bone metastases from solid tumors; and
• treatment of postmenopausal women with osteoporosis at high risk for fracture.21,22
Soluble RAGE mimic is another avenue that is currently under investigation for medical management of Charcot neuroarthropathy. Witzke and colleagues demonstrated that patients with diabetes without Charcot neuroarthropathy had reduced serum levels of sRAGE by 50 percent and those with Charcot neuroarthropathy had 87 percent lower serum levels of sRAGE in comparison to the healthy control group.23 Current investigations are aiming to guage the efficacy of sRAGE ligand decoys. Ramasamy and colleagues deduce that RAGE inhibition would not carry physiological adverse effects or suppress the innate immune system.24
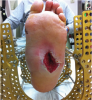 Teriparatide (Forteo, Eli Lilly) is a recombinant form of human parathyroid hormone with FDA approval for osteoporotic men and postmenopausal women at high risk for fractures. Intermittent exposure to teriparatide will activate more osteoblasts than osteoclasts.25 Currently, teriparatide is the only drug approved for anabolic bone growth. However, its use carries potential risks of osteosarcoma development.26
Teriparatide (Forteo, Eli Lilly) is a recombinant form of human parathyroid hormone with FDA approval for osteoporotic men and postmenopausal women at high risk for fractures. Intermittent exposure to teriparatide will activate more osteoblasts than osteoclasts.25 Currently, teriparatide is the only drug approved for anabolic bone growth. However, its use carries potential risks of osteosarcoma development.26
 While the treatment modalities and physiological understanding of Charcot neuroarthropathy may be much different today than in 1827, the presentation has remained relatively the same.1 Classically, Charcot neuroarthropathy presents as a unilateral redness and swelling of a lower extremity. Typically, this increase in warmth precipitates bony changes visible on X-rays and bone scans.
Due to coexisting peripheral neuropathy, only around 30 percent of patients will notice pain or discomfort prior to these changes.2 In our experience, acute trauma is often a common trigger for a Charcot “flare-up.” However, researchers report this in only 22 to 53 percent of patients.3 Currently, Charcot neuroarthropathy is one of the most devastating conditions secondary to diabetes, affecting up to 13 percent of high-risk patients with diabetes mellitus.4
Modern treatment modalities for Charcot foot center on immobilization and offloading either by total contact casts or removable controlled ankle motion (CAM) boot walkers.5 These attempts at preventing further deformity minimize the chance of ulceration leading to amputation. Surgical debridement and episodic reconstructions are also common, despite their high cost and unlikeliness to resolve the causative underlying physiologic mechanisms. These limitations typically keep these treatments from being functionally first-line treatment efforts.
We commonly prescribe adjunctive therapies such as biguanides and bisphosphonates in the acute Charcot osteoarthropathy although current evidence is inconclusive.6,7 Larger clinical trials are still needed to establish the efficacy of pharmaceutical intervention.
While the treatment modalities and physiological understanding of Charcot neuroarthropathy may be much different today than in 1827, the presentation has remained relatively the same.1 Classically, Charcot neuroarthropathy presents as a unilateral redness and swelling of a lower extremity. Typically, this increase in warmth precipitates bony changes visible on X-rays and bone scans.
Due to coexisting peripheral neuropathy, only around 30 percent of patients will notice pain or discomfort prior to these changes.2 In our experience, acute trauma is often a common trigger for a Charcot “flare-up.” However, researchers report this in only 22 to 53 percent of patients.3 Currently, Charcot neuroarthropathy is one of the most devastating conditions secondary to diabetes, affecting up to 13 percent of high-risk patients with diabetes mellitus.4
Modern treatment modalities for Charcot foot center on immobilization and offloading either by total contact casts or removable controlled ankle motion (CAM) boot walkers.5 These attempts at preventing further deformity minimize the chance of ulceration leading to amputation. Surgical debridement and episodic reconstructions are also common, despite their high cost and unlikeliness to resolve the causative underlying physiologic mechanisms. These limitations typically keep these treatments from being functionally first-line treatment efforts.
We commonly prescribe adjunctive therapies such as biguanides and bisphosphonates in the acute Charcot osteoarthropathy although current evidence is inconclusive.6,7 Larger clinical trials are still needed to establish the efficacy of pharmaceutical intervention.
Where The Previous Theories On Charcot Etiology Fall Short
Understanding the biomechanical alterations present in Charcot neuroarthropathy is important for understanding its development. Commonly, neuropathic loss of sensation conjoins with metabolic compromise. This tandem sequelae leads to the musculoskeletal breakdown of pedal architecture. Often, this leads to subluxation of the forefoot and rearfoot at one of many possible locations. The distortion of muscular balance and functional vectors of tendon leverage further exacerbate this progression. There have been two main competing theories behind the etiology of Charcot development. Classically, the neurovascular theory holds that vascular damage to the autonomic nervous system causes a reflex hyperemia and an increase in blood flow to the limbs via arteriovenous shunts.7 The second theory holds that mechanical microtrauma initiates an inflammatory cascade that self-perpetuates into gross bony breakdown.7 However, it is more likely that both of these theories function together as autonomic dysfunction inhibits the perception of repetitive stress amid the accumulation of circulating inflammatory cytokines.
However, neither theory explains why Charcot episodes typically develop unilaterally and occur in only a small percentage of patients with diabetic neuropathy. Also, Charcot can develop secondary to vascular disease, skirting many of the aforementioned pathways.8 Foot and ankle specialists often relate the incidence of inflammation (itself a possible initiator of Charcot episodes) with gross bony changes. At times, this may appear secondary to infection and can be misleading to unfamiliar diagnosticians, such as clinicians at urgent care facilities.
While the mechanisms connecting Charcot’s inflammatory cascade to its phenotypic disease state presentation are wholly unknown, new research in the physiological changes that occur during neuroarthropathic breakdown is unveiling a more quantified disease mechanism. This emerging research could lead to better means of early diagnosis as well as improved medical management.
There have been two main competing theories behind the etiology of Charcot development. Classically, the neurovascular theory holds that vascular damage to the autonomic nervous system causes a reflex hyperemia and an increase in blood flow to the limbs via arteriovenous shunts.7 The second theory holds that mechanical microtrauma initiates an inflammatory cascade that self-perpetuates into gross bony breakdown.7 However, it is more likely that both of these theories function together as autonomic dysfunction inhibits the perception of repetitive stress amid the accumulation of circulating inflammatory cytokines.
However, neither theory explains why Charcot episodes typically develop unilaterally and occur in only a small percentage of patients with diabetic neuropathy. Also, Charcot can develop secondary to vascular disease, skirting many of the aforementioned pathways.8 Foot and ankle specialists often relate the incidence of inflammation (itself a possible initiator of Charcot episodes) with gross bony changes. At times, this may appear secondary to infection and can be misleading to unfamiliar diagnosticians, such as clinicians at urgent care facilities.
While the mechanisms connecting Charcot’s inflammatory cascade to its phenotypic disease state presentation are wholly unknown, new research in the physiological changes that occur during neuroarthropathic breakdown is unveiling a more quantified disease mechanism. This emerging research could lead to better means of early diagnosis as well as improved medical management.
A Closer Look At The Emerging Research On RANK, RANKL, M-CSF And Osteoprotegerin
Emerging treatment options for Charcot aim at targeting the origin and differentiation of osteoclastic activity. Osteoclasts and osteoblasts perform an integral and often inseparable role in the maintenance and remodeling of bone structure. The biochemical receptor activator of nuclear factor kappa-b ligand (RANK/RANKL) regulates the balance between bone deposition and resorption.9 This cytokine is part of the larger tissue necrosis factor alpha (TNF-a) superfamily and is balanced by osteoprotegerin.10 An imbalance of the RANK, RANKL and osteoprotegerin pathways has been closely linked to a number of diseases of bone tissue. The RANK is a membrane receptor on the surface of osteoclast precursor cells. Central to the maturation of osteoclasts from their primal precursors is the binding of RANK with its co-activator RANKL.11 Numerous cells including T-lymphocytes, dendritic cells, endothelial cells, fibroblasts and osteoblasts secrete the RANK ligand. However, only osteoblasts secrete macrophage-colony stimulating factor (M-CSF), an enzyme that stimulates osteoclast precursor cells to express RANK on their surface membranes.9 In this way, the function and secretory cascade of osteoblasts are crucial to the development and maturation of osteoclasts. Osteoblasts can also inhibit the differentiation of osteoclasts by secreting osteoprotegerin, a decoy competitor ligand to RANKL.12 Osteoprotegerin acts as a competitive inhibitor to the RANKL by binding to the RANK surface membrane. Animal studies have demonstrated this to reduce the differentiation and production of osteoclast cells.9,13 Further study on this topic is currently underway and the effects may help reverse or reduce the symptoms of diseases such as osteopenia, osteoporosis, Charcot foot and other bony dysfunctions.
Calcitonin also has an important role in the differentiation of osteoclasts. Calcitonin, released by C cells in the thyroid, has a physiologic role in the body in decreasing serum calcium levels in the body. Calcitonin may also directly inhibit osteoclastic differentiation and activity via calcitonin receptors on osteoclasts.14,15
Osteoblasts can also inhibit the differentiation of osteoclasts by secreting osteoprotegerin, a decoy competitor ligand to RANKL.12 Osteoprotegerin acts as a competitive inhibitor to the RANKL by binding to the RANK surface membrane. Animal studies have demonstrated this to reduce the differentiation and production of osteoclast cells.9,13 Further study on this topic is currently underway and the effects may help reverse or reduce the symptoms of diseases such as osteopenia, osteoporosis, Charcot foot and other bony dysfunctions.
Calcitonin also has an important role in the differentiation of osteoclasts. Calcitonin, released by C cells in the thyroid, has a physiologic role in the body in decreasing serum calcium levels in the body. Calcitonin may also directly inhibit osteoclastic differentiation and activity via calcitonin receptors on osteoclasts.14,15
Current Insights On The RAGE Pathway And Cytokine Recruitment
Significant research has opened new understanding into the contribution of collagen crosslinking and its effects on overall bone strength. Normally, lysyl oxidase acts as an enzyme crosslinking collagen fibrils. Each collagen crosslink created by lysyl oxidase increases the quality and tensile strength of bone. However, non-enzymatic crosslinking results in the formation of advanced glycation end products (AGEs), deteriorating both the biological and mechanical properties of bone.16 Researchers correlate the presence of AGEs in bone collagen with weakened collagen fibers, increased bone fragility and an impaired ability to promote the maturation of preosteoblasts to osteoblasts. Additionally, AGE exposure in connective tissue is linked to osteoblast apoptosis, further withholding a primary repair mechanism from the body’s natural ability to recover from bony lysis and microfractures.17 Carboxymethyllysine is a predominant AGE that accumulates in bone during diabetic complications. This deposition is a process mediated by the RAGE pathway. The RAGE receptor is a pattern recognition receptor for AGEs, which is constitutively expressed but expressed at increased levels during the inflammatory process. Schmidt and colleagues demonstrated that patients with diabetes display elevated carboxymethyllysine levels, byproducts that continue to bind and activate RAGE pathway signaling.18 Advanced glycation end-product interaction with the RAGE receptor creates a chronic cascade of inflammation and tissue injury. Researchers link this to inflammation via the RANKL pathway, further linking it to the proliferation of procytokines such as IL-6, TNF-alpha and free radicals.16 These stimulate the RANKL pathways, leading to enhanced osteoclastogenesis and impaired bone matrix mineralization.16 Soluble RAGE (sRAGE) acts as a competitive antagonist to the RAGE receptors for AGE. The use of sRAGE may prevent AGE binding and the activation of the inflammatory cascade. Serum sRAGE may be elevated in patients with diabetes without severe foot complications and studies have found sRAGE to be reduced in those with the most severe diabetic foot conditions.18 Other research has shown sRAGE concentrations to be decreased in the presence of diabetes mellitus, multiple sclerosis, atherosclerosis, renal failure and as a byproduct of aging. Unfortunately, most of these are also common comorbidities that occur with the progression of diabetes.What You Should Know About Emerging Treatment Options
Surgical strategies for Charcot deformity have evolved over the past several decades. Modern internal and external fixation constructs greatly assist the stability of Charcot reconstruction techniques. Modern imaging and preoperative planning techniques will prove increasingly useful as the prevalence of additive manufacturing (3D printing) technologies increases.19 These techniques have already inexpensively aided patient specific preoperative planning and may greatly increase patient outcomes. However, proper surveillance and early detection are often aberrant. A majority of patients with acute Charcot episodes do not receive treatment or, in many cases, are altogether misdiagnosed. Preventative and therapeutic medical management options are sparse and widespread efforts to increase patient and provider education are still warranted.
Previously, bisphosphonates were among the few drugs known to help acute Charcot patients. However, recent studies validated the use of intranasal calcitonin as an effective alternative for acute Charcot.3,4 More importantly, studies have shown calcitonin to be safe in patients with renal insufficiency, a complication limiting bisphosphonate use.3,4
In 2006, Bem and colleagues showed the efficacy of intranasal calcitonin for decreasing the markers of bone turnover in acute Charcot neuroosteoarthropathy.14 In contrast to bisphosphonates that decrease bone resorption as well as bone formation markers, calcitonin does not decrease bone formation markers. As we mentioned above, osteoclasts have specific calcitonin receptors that bind calcitonin. By directly inhibiting osteoclasts, interventional calcitonin may effectively decrease bone resorption without altering osteoblastic activity.
The RANK ligand inhibitors are human monoclonal antibodies that have affinity and specificity to RANK ligand. Recently, an eight-year study of a phase 2 clinical trial showed denosumab to be a potent inhibitor of bone turnover in postmenopausal women.20 McClung and coworkers showed significant increases in patients’ bone marrow densities and reductions on bone tumor markers.20
Currently, RANKL inhibitors such as denosumab only have FDA approval for the following:
• unresectable giant cell tumors of bone in adults and skeletally mature adolescents;
• increasing bone mass in patients at high risk for fracture including those taking androgen deprivation therapy for nonmetastatic prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer;
• prevention of skeletal-related events in patients with bone metastases from solid tumors; and
• treatment of postmenopausal women with osteoporosis at high risk for fracture.21,22
Soluble RAGE mimic is another avenue that is currently under investigation for medical management of Charcot neuroarthropathy. Witzke and colleagues demonstrated that patients with diabetes without Charcot neuroarthropathy had reduced serum levels of sRAGE by 50 percent and those with Charcot neuroarthropathy had 87 percent lower serum levels of sRAGE in comparison to the healthy control group.23 Current investigations are aiming to guage the efficacy of sRAGE ligand decoys. Ramasamy and colleagues deduce that RAGE inhibition would not carry physiological adverse effects or suppress the innate immune system.24
However, proper surveillance and early detection are often aberrant. A majority of patients with acute Charcot episodes do not receive treatment or, in many cases, are altogether misdiagnosed. Preventative and therapeutic medical management options are sparse and widespread efforts to increase patient and provider education are still warranted.
Previously, bisphosphonates were among the few drugs known to help acute Charcot patients. However, recent studies validated the use of intranasal calcitonin as an effective alternative for acute Charcot.3,4 More importantly, studies have shown calcitonin to be safe in patients with renal insufficiency, a complication limiting bisphosphonate use.3,4
In 2006, Bem and colleagues showed the efficacy of intranasal calcitonin for decreasing the markers of bone turnover in acute Charcot neuroosteoarthropathy.14 In contrast to bisphosphonates that decrease bone resorption as well as bone formation markers, calcitonin does not decrease bone formation markers. As we mentioned above, osteoclasts have specific calcitonin receptors that bind calcitonin. By directly inhibiting osteoclasts, interventional calcitonin may effectively decrease bone resorption without altering osteoblastic activity.
The RANK ligand inhibitors are human monoclonal antibodies that have affinity and specificity to RANK ligand. Recently, an eight-year study of a phase 2 clinical trial showed denosumab to be a potent inhibitor of bone turnover in postmenopausal women.20 McClung and coworkers showed significant increases in patients’ bone marrow densities and reductions on bone tumor markers.20
Currently, RANKL inhibitors such as denosumab only have FDA approval for the following:
• unresectable giant cell tumors of bone in adults and skeletally mature adolescents;
• increasing bone mass in patients at high risk for fracture including those taking androgen deprivation therapy for nonmetastatic prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer;
• prevention of skeletal-related events in patients with bone metastases from solid tumors; and
• treatment of postmenopausal women with osteoporosis at high risk for fracture.21,22
Soluble RAGE mimic is another avenue that is currently under investigation for medical management of Charcot neuroarthropathy. Witzke and colleagues demonstrated that patients with diabetes without Charcot neuroarthropathy had reduced serum levels of sRAGE by 50 percent and those with Charcot neuroarthropathy had 87 percent lower serum levels of sRAGE in comparison to the healthy control group.23 Current investigations are aiming to guage the efficacy of sRAGE ligand decoys. Ramasamy and colleagues deduce that RAGE inhibition would not carry physiological adverse effects or suppress the innate immune system.24
 Teriparatide (Forteo, Eli Lilly) is a recombinant form of human parathyroid hormone with FDA approval for osteoporotic men and postmenopausal women at high risk for fractures. Intermittent exposure to teriparatide will activate more osteoblasts than osteoclasts.25 Currently, teriparatide is the only drug approved for anabolic bone growth. However, its use carries potential risks of osteosarcoma development.26
Teriparatide (Forteo, Eli Lilly) is a recombinant form of human parathyroid hormone with FDA approval for osteoporotic men and postmenopausal women at high risk for fractures. Intermittent exposure to teriparatide will activate more osteoblasts than osteoclasts.25 Currently, teriparatide is the only drug approved for anabolic bone growth. However, its use carries potential risks of osteosarcoma development.26











