Current Insights On Bioengineered Alternative Tissues
Bioengineered alternative tissues (BATs) can be valuable advanced treatments for wounds that are not healing. These expert panelists share their clinical experience with using BATs, appropriate timing for considering BATs and what wounds they most commonly use BATs to treat.
Q:
In which wounds do you most commonly use BATs?
A:
For Paul Kim, DPM, MS, the use of BATs depends on the specific situation and not necessarily the wound etiology. He says one should reserve dermoinductive agents (i.e. cell-based therapies) for wounds that are not healing at a measurable rate, typically a rate of 15 percent per week.1 Dr. Kim notes the treatment plan should always include maximizing perfusion, medical and nutrition optimization, offloading (via device and/or surgical), regular clinic or operative excisional debridement, compression, and infection/biofilm control. If these measures fail to improve the wound healing, he says one can consider dermoinductive agents. 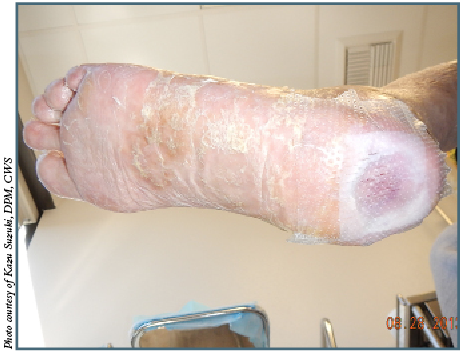
Clinicians should utilize dermoconductive agents (tissue scaffolds) to create a neodermis for deeper soft tissue defects, according to Dr. Kim. As he notes, this neodermis is necessary to create a durable base to cover deeper, exposed tissues such as muscle, tendon and fascia. Once a neodermis forms, Dr. Kim says one can apply split thickness skin grafts (STSGs), what he considers the “gold standard,” to heal the wound. He notes that physicians should apply dermoconductive agents in the OR after performing excisional debridement.
David G. Armstrong, DPM, MD, PhD, considers biologics/skin substitutes for wounds already simplified by vertically reducing the depth of the wound. This is part of his “vertical” and “horizontal” strategies for healing.2,3
Kazu Suzuki, DPM, CWS, utilizes about six different brands of BATs for all kinds of wounds. He uses a lot of BATs daily in his wound care center, except for superficial wounds like skin tears. By the application numbers, he notes venous leg ulcers receive the most BATs, followed by diabetic foot ulcers and then all the other types of wounds including pressure, surgical and burn wounds.
“We do believe (BATs) are indispensable in closing chronic and difficult to heal wounds, especially in diabetic foot ulcers as well as the wounds of compromised patients (cancer patients, dialysis patients, etc.),” says Dr. Suzuki.
Peter Blume, DPM, uses BATs on wounds that are recalcitrant to common offloading and advanced moist wound therapies. He does consider BATs for granular wounds that may need additional growth factors in order to stimulate epithelialization. The active therapies with living skin equivalents may provide some beneficial effects for granular wounds, according to Dr. Blume.
Q:
Are there any clinical scenarios in which you would consider using BATs earlier than the oft-quoted one month of failed standard wound care?1
A:
Although every BAT product has its own indication, Dr. Suzuki thinks it is reasonable to wait until after four to six weeks of supervised medical care (as opposed to self-care) before considering the use of BATs. To have the best outcome, he says the clean wound bed should be in the best condition possible as BATs are expensive and often limited in how many one can apply to a single wound. He says this means it will take a few weeks of good wound debridement after the first visit to the clinic. In Dr. Suzuki’s wound care center, most of the patients come from referrals (from internists, dermatologists or other surgeons) so he notes almost all of them do qualify for BAT use after having four weeks of failed or unsatisfactory local wound care treatment.
Sometimes, Dr. Suzuki would like to use STSG to close a wound but cannot harvest skin graft because a patient refuses the harvesting procedure, or more likely does not have good quality skin because of extreme age, which happens with elderly patients. Besides using BATs in this case, he cites the CelluTome Epidermal Harvesting System (KCI) as a new device that allows one to harvest epidermal grafts and says it may provide an alternative to STSGs in these selected cases.
For deeper soft tissue defects, Dr. Kim says one can apply dermoconductive agents after excisional debridement in the operating room regardless of wound chronicity. He emphasizes that rapid coverage or closure of deeper exposed tissues is important to prevent further progression of wound size and infection.
“It is not clinically or economically prudent to repeatedly apply any product without giving thought to a definitive closure plan as well as addressing wound care fundamentals,” adds Dr. Kim. “Utilizing BAT products as first-line treatments is misguided in the current cost-conscious healthcare environment.”
Q:
In your clinical experience, are there any BATs that work better than others in certain wounds?
A:
At this point, nobody knows that answer, says Dr. Suzuki. He is “eagerly waiting to see a head-to-head comparison” of the different BATs to see whether one product is superior over the others. Having said that, Dr. Suzuki notes such a study would be hard to control and costly, and we may never see a direct comparison study among BATs. Dr. Kim concurs. To date, there are no meaningful head-to-head, unbiased, efficacy or effectiveness clinical trials comparing one BAT product to another, according to Dr. Kim. He adds that all BAT products will fail if the fundamentals of wound care treatment go unaddressed.
While various types of biologics are helpful, Dr. Armstrong notes his most frequent go-to therapy following “vertical” wound simplification is a STSG. He cites research showing this can be a helpful adjunct.4 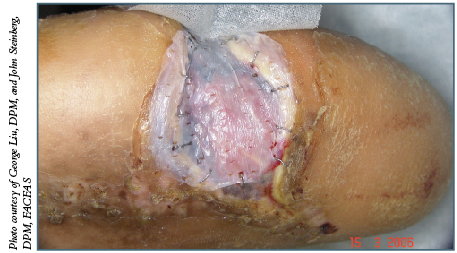
Dr. Suzuki generally recommends becoming familiar with several different brands of grafts and using them as one sees fit as all BATs handle differently and have different wound indications, and no single product works universally well for all etiologies of lower extremity wounds. Although BATs come from different tissue origins (from living human cells, amniotic membrane, cadaveric human skin and various animal tissues), Dr. Suzuki notes collagen structures are similar among various BATs. Accordingly, he speculates it may be difficult to show meaningful clinical differences in patients. Dr. Suzuki agrees with Dr. Armstrong that STSG is useful in all wound types when BATs are not available.
Dr. Armstrong is a Professor of Surgery and Director of the Southern Arizona Limb Salvage Alliance (SALSA) at the University of Arizona College of Medicine.
Dr. Blume is an Assistant Clinical Professor of Surgery in the Department of Surgery and an Assistant Clinical Professor of Orthopaedics and Rehabilitation in the Department of Orthopaedics, Section of Podiatric Surgery at the Yale University School of Medicine in New Haven, Ct. Dr. Blume is a Fellow of the American College of Foot and Ankle Surgeons.
Dr. Kim is an Associate Professor at Georgetown University School of Medicine in Washington, DC. He is a Fellow of the American College of Foot and Ankle Surgeons.
Dr. Suzuki is the Medical Director of the Tower Wound Care Center at the Cedars-Sinai Medical Towers. He is also on the medical staff of the Cedars-Sinai Medical Center in Los Angeles and is a Visiting Professor at the Tokyo Medical and Dental University in Tokyo. Dr. Suzuki can be reached via e-mail at Kazu.Suzuki@CSHS.org .
References
1. Sheehan P, Jones P, Giurini JM, et al. Percent change in wound area of diabetic foot ulcers over a four-week period is a robust predictor of complete healing in a 12-week prospective trial. Plast Reconstr Surg. 2006; 117(7 Suppl):239S-244S.
2. Isaac AL, Armstrong DG. Negative pressure wound therapy and other new therapies for diabetic foot ulceration: the current state of play. Med Clin North Am. 2013; 97(5):899-909.
3. Pappalardo J, Plemmons B, Armstrong DG. Wound healing simplification: a vertical and horizontal philosophy illustrated. J Wound Technology. 2013; 19:38-39.
4. Rose JF, Giovinco N, Mills JL, Armstrong DG. Split-thickness skin grafting in the high-risk diabetic foot. J Vasc Surg. 2014; 59(6):1657-63.











