Current Concepts In Vascular Imaging
 Given the increased incidence of peripheral arterial disease and critical limb ischemia, it is essential to have a firm grasp of diagnostic imaging techniques and facilitate appropriate referrals when necessary. These authors assess the strengths and weaknesses of noninvasive vascular imaging modalities, and discuss the emergence of alternatives such as CO2 digital subtraction angiography.
Given the increased incidence of peripheral arterial disease and critical limb ischemia, it is essential to have a firm grasp of diagnostic imaging techniques and facilitate appropriate referrals when necessary. These authors assess the strengths and weaknesses of noninvasive vascular imaging modalities, and discuss the emergence of alternatives such as CO2 digital subtraction angiography.
Peripheral arterial disease (PAD) has been increasing in prevalence, affecting 12 to 20 percent of patients worldwide.1 The most severe form is critical limb ischemia (CLI), which is characterized by rest pain, ischemic ulceration and gangrene.2 An estimated 40 percent of CLI patients without revascularization will experience a major amputation within a year of the development of CLI.3
Revascularization plays a major role in preventing amputation as well as improving quality of life and prolonging survival.4 Accordingly, it falls on the shoulders of vascular surgeons, podiatrists and other multidisciplinary specialists to diagnose and manage PAD effectively through serial evaluations.5,6
The process begins by a history taking and physical examination of patients with lower extremity wounds. However, patient history and physical exam alone cannot always evaluate the severity of the disease process or effectively guide the need for therapeutic intervention. All PAD patients with some form of CLI require objective testing. Both noninvasive and invasive vascular testing are extensions of the vascular history and physical examination. Clinicians can use this testing to confirm a diagnosis of arterial disease and determine the level and extent of disease. There have been recent improvements in noninvasive vascular imaging including computed tomography angiography (CTA), magnetic resonance angiography (MRA) and conventional angiography.
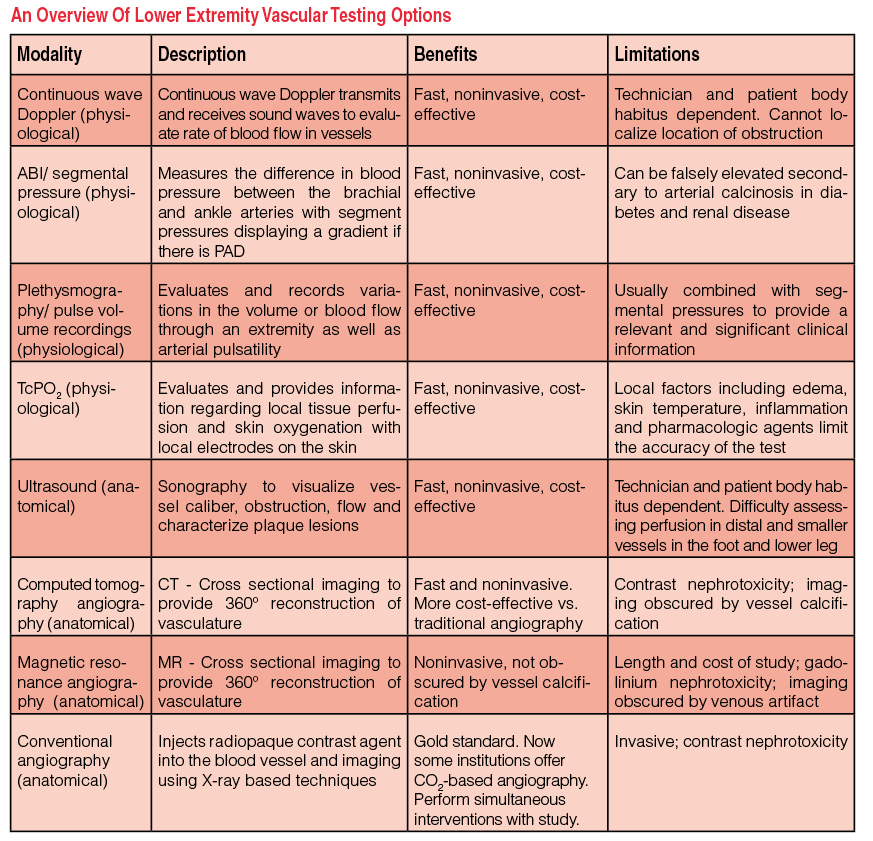 Given all those improvements, all specialists caring for patients with PAD should have a working knowledge of the many available modalities to order the appropriate diagnostic test for their patients (see “An Overview of Lower Extremity Vascular Testing Options” at right). One should initially refer patients with CLI for noninvasive vascular imaging prior to invasive conventional angiography when planning limb salvage surgery.
Given all those improvements, all specialists caring for patients with PAD should have a working knowledge of the many available modalities to order the appropriate diagnostic test for their patients (see “An Overview of Lower Extremity Vascular Testing Options” at right). One should initially refer patients with CLI for noninvasive vascular imaging prior to invasive conventional angiography when planning limb salvage surgery.
Available studies include physiologic tests such as the ankle-brachial index (ABI), plethysmography, pulse volume recordings (PVR) and transcutaneous oxygen monitoring, which correlate symptoms with the site and severity of arterial occlusive disease. In the clinical setting, the use of noninvasive vascular testing assesses the severity of arterial insufficiency in affected extremities. Physicians have supplemented these tests in recent years with anatomical imaging modalities such as ultrasound, MRA and CTA, which are more sensitive in identifying vascular disease process in the lower extremities. Finally, digital subtraction angiography has become an indispensable tool and the gold standard for early detection and treatment of PAD to avoid preventable complications and additional interventions.
Recognizing The Benefits And Limitations Of The ABI
 The preliminary assessment with noninvasive imaging begins with an ABI, a simple, fast and cost-effective method to evaluate PAD. Clinicians can perform the ABI in the office or at the bedside.7 One does so by determining the systolic blood pressure in both brachial arteries and then in both the dorsalis pedis and posterior tibial arteries.5 Then calculate for each lower extremity and interpret this as an ABI value (see the table “How To Interpret ABI Readings” at left). A normal ABI is a value greater than 0.9. An ABI value of 0.5 to 0.9 is characteristic of PAD while a value of less than 0.5 suggests CLI as well as severe multi-vessel disease.8 In patients with suspected PAD but normal resting ABIs, exercise testing of ABI can occur.
The preliminary assessment with noninvasive imaging begins with an ABI, a simple, fast and cost-effective method to evaluate PAD. Clinicians can perform the ABI in the office or at the bedside.7 One does so by determining the systolic blood pressure in both brachial arteries and then in both the dorsalis pedis and posterior tibial arteries.5 Then calculate for each lower extremity and interpret this as an ABI value (see the table “How To Interpret ABI Readings” at left). A normal ABI is a value greater than 0.9. An ABI value of 0.5 to 0.9 is characteristic of PAD while a value of less than 0.5 suggests CLI as well as severe multi-vessel disease.8 In patients with suspected PAD but normal resting ABIs, exercise testing of ABI can occur.
Obtain a baseline ABI prior to exercise. Have the patient exercise, usually by walking on a treadmill at a constant speed for five minutes or until claudication onset with re-measurement of the ankle pressures afterward. A decrease in ABI of 15 to 20 percent would be diagnostic of PAD.
Patients with medial arteriosclerosis may have falsely elevated ABIs so the clinician should consider evaluating toe pressures with or in lieu of ankle pressures to obtain a toe brachial index (TBI). A TBI greater than 0.75 is considered normal while a value of less than 0.25 is consistent with severe PAD. Patients with toe pressures less than 55 mmHg are unlikely to heal lower extremity ulcers.9
By performing segmental pressure evaluation on individual segments of a limb, one can determine the location of a focal lesion. A gradient greater than 20 mmHg indicates significant disease in the intervening segment.10
Accordingly, we recommend ABI measurement as an initial diagnostic test for patients with PAD as the ABI can assess the healing potential of foot lesions with some success. However, in the presence of medial arteriosclerosis or diabetes mellitus, the arteries are poorly compressible and the ABI may give falsely elevated values that will not evaluate the state of microvasculature.8,9
Continuous wave Doppler measures the flow velocity in arteries. One would place a handheld Doppler probe over the skin, overlying the course of the blood vessel under examination. The probe emits a single frequency sound wave while the receiving element continuously detects any echoes from the sensitive region of the beam. The transmitted sound waves strike moving blood cells and reflect back to the receiving element of the Doppler probe. An amplifier filters the sound and gives frequency tracings. The velocity of blood flow is proportional to the frequency shift in sound waves that transmit back to the receiver.
One then grades the Doppler signal received with regard to strength and phasicity. With a normal triphasic signal, there would be normal arterial flow that is usually associated with a palpable pulse. A biphasic signal indicates increasing stenosis with mild to moderate PAD. Finally, in advanced PAD (with severe stenosis), the signal will become monophasic. One can best utilize this modality in conjunction with other techniques such as ABI. A normal ABI value in the face of a monophasic Doppler signal or flattened Doppler waveform indicates the ABI is falsely elevated. Falsely elevated ABIs are relatively common in patients with diabetes because the presence of calcification in the arterial wall (medial calcinosis) makes the artery noncompressible.11
Key Pointers On Plethysmography And Pulse Volume Recordings
Plethysmography is an important adjunct that uses the principle of volume change in a body region to measure blood flow in the targeted extremity. A plethysmograph is a device that records variations in the volume through an extremity. The volume of an extremity changes between systole and diastole as a result of the pulsatile blood flow into that region.
The most popular modalities are air plethysmography and photoplethysmography. Pulse volume recording (PVR) is a form of air plethysmography, in which one usually obtains lower extremity pulsatility as an indirect measurement of arterial flow along with plethysmography. The normal PVR is characterized by a sharp upstroke (anacrotic slope), a distinct pulse peak and rapid decline (catacrotic slope). In patients with PAD, arterial stenosis becomes progressively flattened and prolonged.
The value of the PVR test is that medial wall calcification does not affect it and is therefore quite useful in the diabetic population.12 The PVR tracings at the foot level have also been an indicator of the healing potential of foot procedures.13 Pulse volume recordings are most useful when one combines them with ABIs and segmental pressures to provide a more complete assessment of limb blood flow. The PVR is a functional test that assesses the sum of all blood flow to the examined limb (global perfusion) but will not examine specific blood vessels. It does, however, allow us to correlate examination findings and in many cases identify the affected regions (with stenosis or occlusions).
What You Should Know About Transcutaneous Oxygen Monitoring
Transcutaneous oxygen monitoring, more specifically transcutaneous partial pressure of oxygen (TcPO2) measurement, provides information regarding local tissue perfusion and skin oxygenation.
Clinicians can perform the test with platinum oxygen electrodes on the patient’s chest wall and legs or feet. One can use the absolute value of the oxygen tension at the foot or leg, or a ratio of the foot value to chest wall value.14 A TcPO2 value of >40 mmHg correlates with wound healing while values <20 mmHg indicate a poor prognosis. Clinicians can do this test easily in the office or a clinic setting, and it is cost-effective. The main drawbacks are that local factors including edema, skin temperature, inflammation and pharmacologic agents limit the accuracy of this test.
Examining The Research On Duplex Ultrasound Imaging
Duplex ultrasound imaging incorporates two elements: gray-scale ultrasonography (B-mode imaging) and color-flow Doppler (using pulsed Doppler spectral analysis).
One can use duplex ultrasound in noninvasive vascular imaging to evaluate vessel structure, stenotic segments and occlusions, and identify the morphology of an atherosclerotic plaque. Color-flow ultrasound Doppler can visualize the blood flow within a blood vessel. This noninvasive method provides clinically useful hemodynamic information in patients with suspected aortoiliac occlusive disease by accurately detecting and grading lesions in the aortoiliac region. The use of duplex ultrasound can effectively diagnose the presence and extent of arterial disease without the need for arteriography.15,16
Rosfor and colleagues evaluated 60 limbs from 73 patients and found there was 92 percent (194/211) agreement between duplex ultrasound and angiography in the grading and identification of lesions and arterial segments.15 De Vos and colleagues evaluated duplex ultrasound versus computed tomography angiography for treatment planning in 12 patients. After formulating the initial 36 treatment plans with duplex ultrasound, authors noted that an additional CTA study confirmed 27 (75 percent) treatment plans, changed six (17 percent) plans and supplemented three (8 percent) plans. Peripheral arterial disease treatment planning based on CTA was mostly consistent with duplex ultrasound-based treatment plans although the authors still felt CTA was needed to confirm their interventional strategy.17
Unfortunately, duplex ultrasound cannot evaluate the microvasculature and perfusion in the lower extremity. Accordingly, this modality cannot effectively predict or monitor wound healing in patients with CLI. It also has limited depth penetration and is only useful in the assessment of macrovessel structures and blood flow. Additionally, as a modality, duplex ultrasound depends on the skill level of the ultrasound technician. Other issues include patient body habitus as an obese patient may have poor results when evaluating aortoiliac and iliofemoral vessels due to poor penetration.
Duplex ultrasound remains a popular noninvasive modality for surveillance after a revascularization procedure to monitor patency for both grafts and native vessels. Overall, duplex ultrasound is a safe, fast, noninvasive study that is relatively easy to perform in a variety of settings.
How Effective Is Computed Tomography Angiography?
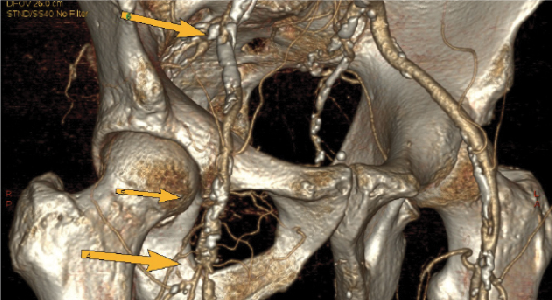 Computed tomography angiography with multidetector scanners has increasingly been in favor over traditional angiography with digital subtraction (see left photo). Met and coworkers performed a systemic review and meta-analysis evaluating the diagnostic accuracy of CTA for the detection of more than 50 percent arterial stenosis.18 They found the sensitivity and specificities for the aortoiliac, femoropopliteal and tibial arteries were 96 and 98, 97 and 94, 95 and 91 percent respectively. There was a slight overall decrease in both the sensitivity (92 versus 97 percent) and specificity (93 versus 98 percent) when evaluating images from 2-4 slice multidetector CTA imaging versus 16-64 slice multidetector CTA imaging respectively.
Computed tomography angiography with multidetector scanners has increasingly been in favor over traditional angiography with digital subtraction (see left photo). Met and coworkers performed a systemic review and meta-analysis evaluating the diagnostic accuracy of CTA for the detection of more than 50 percent arterial stenosis.18 They found the sensitivity and specificities for the aortoiliac, femoropopliteal and tibial arteries were 96 and 98, 97 and 94, 95 and 91 percent respectively. There was a slight overall decrease in both the sensitivity (92 versus 97 percent) and specificity (93 versus 98 percent) when evaluating images from 2-4 slice multidetector CTA imaging versus 16-64 slice multidetector CTA imaging respectively.
One consideration with CTA imaging is the use of iodinated contrast material for visualization of the vasculature. There is also the risk of contrast-induced acute kidney injury due to iodinated contrast media. This risk is most pronounced when patients have preexisting renal impairment, especially due to diabetic nephropathy. Other risk factors for contrast-induced acute kidney injury include salt depletion and dehydration; congestive heart failure; advanced age (over 70 years); and concurrent use of a nephrotoxic drug.19,20
Injection rates vary depending on protocol but typically are in the range of 4 to 6 mL/s delivered intravenously by a dual channel power injector. The amount of iodinated media will vary depending upon the patient and protocol one uses with approximately 100–120 mL in use for an abdominal CTA with runoffs. Since the last volume of the bolus will not contribute to the enhancement when scanning below the knees, the injection duration can be shorter by five seconds (e.g., use a 35-second injection time for an acquisition of 40 seconds). However, to ensure the enhancement of all arteries, the injection duration should not be shorter than 30 seconds and in fast scan protocols, one should add an appropriate delay time to prevent outrunning the contrast bolus.18,21
However, the reliability of CTA does suffer when discerning heavily calcified vessel walls from IV contrast, making interpretation sometimes challenging. Another limitation of CTA relates to the significant artifact caused by any metal prostheses, making the study limited in its usefulness. Another issue is direct evidence from epidemiologic studies that the organ doses corresponding to a common CTA study (two or three scans, resulting in a dose in the range of 30 to 90 mSv) result in an increased risk of cancer.21
Overall, multidetector CTA is an emerging non-invasive modality that enables one to assess vasculature including the coronary artery anatomy. In comparison to conventional angiography or duplex ultrasound, multidetector CTA is less expensive, less invasive and faster.18
What You Should Know About Magnetic Resonance Angiography
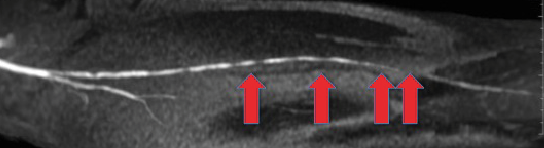 Magnetic resonance angiography is another less invasive alternative to traditional angiography (see right photo). This modality has become more prevalent as the technology and relevant gadolinium contrast protocols have improved.
Magnetic resonance angiography is another less invasive alternative to traditional angiography (see right photo). This modality has become more prevalent as the technology and relevant gadolinium contrast protocols have improved.
The reported sensitivity of 94 percent and specificity of 90 percent of MRA in detecting hemodynamically significant lesions in peripheral vasculature is similar to the CTA imaging technique.10 Baum and colleagues performed a blinded, prospective multicenter study of 155 consecutive patients with either rest pain or tissue loss, comparing MRA versus traditional contrast angiography.22 They found that MRA and contrast angiography are approximately equivalent in diagnostic accuracy. Sensitivity in distinguishing patent segments from completely occluded segments was 83 percent for CTA and 85 percent for MRA, and both had 81 percent specificity. For distinguishing near normal segments (suitable as bypass graft termini), CTA was less sensitive than MRA (77 percent versus 82 percent), but more specific (92 percent versus 84 percent). Meissner and coworkers found similar efficacy comparing MRA versus digital subtraction angiography when evaluating the lower leg and foot.23
In comparison to CTA, vessel wall calcification will not cause an artifact on MRA as it shows the natural contrast between blood and the vessel wall. Other advantages are the absence of ionizing radiation and the diminished nephrotoxicity of gadolinium in comparison to iodinate contrast.22 There are many restrictions (such as implanted pacemakers, metallic prosthesis, cardiac life support systems or history of claustrophobia) that would make patients unsuitable candidates for MRA. Magnetic resonance angiography suffers from increased costs and time to perform the study in comparison to CTA. Magnetic resonance angiography imaging also has difficulty when there is a previous metallic stent in the vessel that could falsely appear stenotic or occluded. Researchers have recently associated magnetic resonance angiography with renal failure and systemic nephrogenic sclerosis.23 The modality is also very expensive and not widely available.23
Evaluating Conventional Angiography And The Emergence Of Digital Subtraction Angiography
Physicians can employ non-invasive modalities such as CTA, MRA and duplex ultrasound to evaluate lower extremity disease, and they provide consistent qualitative information that is on par with conventional angiography while being noninvasive. Despite those advances and increasing availability, with the advent of endovascular therapies becoming first-line treatment, conventional angiography remains the gold standard in mapping the extent and location of arterial pathology prior to revascularization procedures.24
The physician performing conventional angiography typically accesses the contralateral femoral vessel via a percutaneous approach utilizing a vascular catheter. One would use fluoroscopy to direct the wire-guided catheter to the target vessel before injecting contrast material to identify flow throughout the vessel as well as stenosis or obstructing lesions. During the same diagnostic procedure, physicians could perform endovascular therapies such as angioplasty or stent placement on those identified target lesions.24
However, using conventional iodinated contrast media with digital subtraction angiography continues to be associated with small but significant risks of contrast-induced nephrotoxicity and allergic reactions. Elderly patients presenting with PAD often have coexisting renal, cardiac and other medical illnesses that further increase the risk of these complications. Accordingly, in many patients, much of the risk associated with the management of PAD is due to the iodinated contrast arteriography itself.
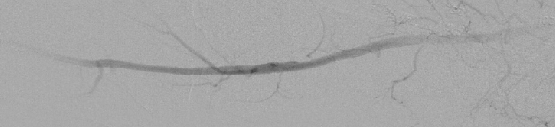 There is growing interest in CO2 digital subtraction angiography as an alternative to traditional angiography. Injection of this CO2 gas with its decreased radiodensity creates radiographic contrast by transiently displacing blood from the artery one is imaging. Carbon dioxide is 20 times more soluble in blood than oxygen and dissolves rapidly by elimination through the lungs during normal respiration.25,26 Intravascular CO2 produces no allergic reaction and causes no nephrotoxicity (see photo at left).
There is growing interest in CO2 digital subtraction angiography as an alternative to traditional angiography. Injection of this CO2 gas with its decreased radiodensity creates radiographic contrast by transiently displacing blood from the artery one is imaging. Carbon dioxide is 20 times more soluble in blood than oxygen and dissolves rapidly by elimination through the lungs during normal respiration.25,26 Intravascular CO2 produces no allergic reaction and causes no nephrotoxicity (see photo at left).
Madhusudhan and colleagues studied 21 consecutive patients (with ages ranging between 29 to 79 with a mean of 48 years) with 27 angiograms (15 unilateral, six bilateral), using both iodinated contrast media and then CO2 as contrast agents during a 15-month period.27 All patients clinically suffered from calf or gluteal claudication and nine patients also had rest pain. The researchers also included patients in the study who had either ischemic foot ulcers or gangrenous changes in the limb.
Of the 195 major named arteries evaluated (common, external and internal iliac, superficial and deep femoral, popliteal, and anterior and posterior tibial), the use of iodinated contrast media opacified 188 (96.4 percent) arteries and CO2 opacified 168 (86.2 percent) arteries. This excluded segments that were not adequately opacified either due to the presence of arterial sheath or complete proximal occlusion. A total of 113 arterial segments of 27 limbs had two evaluations each. Researchers gave a score of 0, 1 or 2 for non-visualization of collaterals, visualization of few collaterals and visualization of many collaterals respectively. Madhusudhan and coworkers found that both techniques graded 180 (79.7 percent) segments the same. Iodinated contrast media evaluated 35 (15.5 percent) segments better and CO2 digital subtraction angiography demonstrated 11 (4.9 percent) segments more clearly.27
Overall, one can obtain appropriate opacification of the iliac and femoral arteries via CO2 angiography and treating physicians should consider CO2 as the initial contrast agent for the evaluation of PAD in patients with renal failure and iodine contrast allergy. If there is inadequate depiction of a vessel despite selective injection, one may supplement this with some amount of iodinated contrast media for better visualization if indicated at that time.
A Closer Look At Future Imaging Options
The aforementioned modalities evaluate the global perfusion of the lower extremity by measuring physiological markers or anatomical structures, and assessing the overall condition of large and medium caliber blood vessels. There are new, upcoming modalities to evaluate microperfusion in the lower extremity in depth with growing interest in targeted revascularization for regional areas of ischemia, the angiosome model of the lower extremity.28
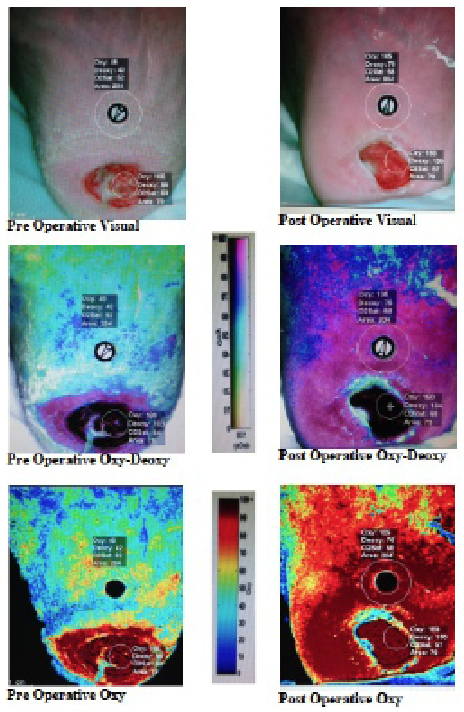 The first emerging modality is hyperspectral imaging, which utilizes scanning spectroscopy to construct spatial maps for tissue oxygenation using wavelengths (between 500-660 nm) of visual light (see photo at right). Hyperspectral imaging offers a noninvasive approach of assessing the success of revascularization and predicting the likelihood of wound failure.29
The first emerging modality is hyperspectral imaging, which utilizes scanning spectroscopy to construct spatial maps for tissue oxygenation using wavelengths (between 500-660 nm) of visual light (see photo at right). Hyperspectral imaging offers a noninvasive approach of assessing the success of revascularization and predicting the likelihood of wound failure.29
Single photon emission computed tomography/positron emission tomography (SPECT/PET) imaging is another variant that utilizes a combination of high sensitivity radiotracer-based imaging with high resolution CT scan imaging that obtains both functional and structural information to better visualize perfusion of ischemic tissues. The main drawbacks would be exposure to ionizing radiation and the costs of maintaining nuclear technicians and producing radioisotopes for daily testing, limiting the availability of this option at smaller hospitals.30
In Conclusion
Although conventional angiography with digital subtraction angiography remains the gold standard and has experienced a resurgence in popularity thanks to endovascular interventions, noninvasive imaging standards are slowly supplanting it in clinical practice. These noninvasive modalities include CTA, MRA and duplex ultrasound. With the advent of multi-detector CT scanners and 3D rendering, MRI devices with developed timing protocols have produced comparative quality imaging that has been useful in planning and deciding the timing of surgical intervention. Duplex ultrasound remains an effective surveillance tool for the post-procedure stage.
On the future horizon, vascular specialists are slowly adopting a new crop of noninvasive imaging as specialists refine their technique in treatment from a global perspective to a regional or microperfusion perspective.28 This will enable vascular and foot specialists to move toward achieving a significant reduction in persistent ulceration and decreasing the rate of amputation in patients with PAD and CLI.
Dr. Benitez is a Vascular Research Fellow at Yale University.
Dr. Sumpio is a Professor of Surgery and Radiology at Yale University.
References
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012; 125(1):e2-e220.
- Novo S, Coppola G, Milio G. Critical limb ischemia: definition and natural history. Curr Drug Targets Cardiovasc Haematol Disord. 2004; 4(3):219-25.
- Sumpio BE. Foot ulcers. N Engl J Med. 2000; 343(11):787-93.
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007. 33(Suppl 1):S1-75.
- Chin JA, Sumpio BE. Diabetes mellitus and peripheral vascular disease: diagnosis and management. Clin Podiatr Med Surg. 2014; 31(1):11-26.
- Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003; 20(4):689-708.
- McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000; 32(6):1164-71.
- Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA. 1969; 207(10):1869-74.
- Williams DT, Price P, Harding KG. The influence of diabetes and lower limb arterial disease on cutaneous foot perfusion. J Vasc Surg. 2006; 44(4):770-5.
- Koelemay MJ, Lijmer JG, Stoker J, et al. Magnetic resonance angiography for the evaluation of lower extremity arterial disease: a meta-analysis. JAMA. 2001; 285(10):1338-45.
- Smith, FB, Lee AJ, Price JF, et al. Changes in ankle brachial index in symptomatic and asymptomatic subjects in the general population. J Vasc Surg. 2003; 38(6):1323-30.
- Darling RC, Raines JK, Brener BJ, Austen WG. Quantitative segmental pulse volume recorder: a clinical tool. Surgery. 1972; 72(6):873-7.
- Gibbons, GW, Wheelock FC Jr., Siembieda C, et al. Noninvasive prediction of amputation level in diabetic patients. Arch Surg. 1979; 114(11):1253-7.
- White RA, Nolan L, Harley D, et al. Noninvasive evaluation of peripheral vascular disease using transcutaneous oxygen tension. Am J Surg. 1982; 144(1):68-75.
- Rosfors S, Eriksson M, Hoglund N, Johansson G. Duplex ultrasound in patients with suspected aorto-iliac occlusive disease. Eur J Vasc Surg. 1993. 7(5):513-7.
- Moneta GL, Yeager RA, Lee RW, Porter JM. Noninvasive localization of arterial occlusive disease: a comparison of segmental Doppler pressures and arterial duplex mapping. J Vasc Surg. 1993; 17(3):578-82.
- De Vos MS, Bol BJ, Gravereaux RC, et al. Treatment planning for peripheral arterial disease based on duplex ultrasonography and computed tomography angiography: consistency, confidence and the value of additional imaging. Surgery. 2014; 156(2):492-502.
- Met R, Bipat S, Legemate DA, et al. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009; 301(4):415-24.
- Andreucci M, Faga T, Pisani A, et al. Acute kidney injury by radiographic contrast media: pathogenesis and prevention. Biomed Res Int. 2014; epub Aug. 14.
- Thomsen HS, Morcos SK. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003; 76(908):513-8.
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007; 357(22):2277-84.
- Baum RA, Rutter CM, Sunshine JH, et al. Multicenter trial to evaluate vascular magnetic resonance angiography of the lower extremity. American College of Radiology Rapid Technology Assessment Group. JAMA. 1995; 274(11):875-80.
- Meissner OA, Rieger J, Weber C, et al. Critical limb ischemia: hybrid MR angiography compared with DSA. Radiology. 2005; 235(1):308-18.
- Hodgson K. Principles of Arteriography. In (Rutherford EB, ed.) Rutherford’s Vascular Surgery, Fifth Edition. WB Saunders, Philadelphia, 2000.
- Kerns SR, Hawkins Jr. IF, Sabatelli FW. Current status of carbon dioxide angiography. Radiol Clin North Am. 1995; 33(1):15-29.
- Hawkins, IF, Caridi JG. Carbon dioxide (CO2) digital subtraction angiography: 26-year experience at the University of Florida. Eur Radiol. 1998; 8(3):391-402.
- Madhusudhan KS, Sharma S, Srivastava DN, et al. Comparison of intra-arterial digital subtraction angiography using carbon dioxide by ‘home made’ delivery system and conventional iodinated contrast media in the evaluation of peripheral arterial occlusive disease of the lower limbs. J Med Imaging Radiat Oncol. 2009; 53(1):40-9.
- Sumpio BE, Forsythe RO, Ziegler KR, et al. Clinical implications of the angiosome model in peripheral vascular disease. J Vasc Surg. 2013; 58(3):814-26.
- Chin JA, Wang EC, Kibbe MR. Evaluation of hyperspectral technology for assessing the presence and severity of peripheral artery disease. J Vasc Surg. 2011; 54(6):1679-88.
- Stacy MR, Zhou W, Sinusas AJ. Radiotracer imaging of peripheral vascular disease. J Nucl Med. 2013; 54(12):2104-10.
For further reading, see “Emerging Vascular Interventions In The Diabetic Foot” in the March 2012 issue of Podiatry Today.











