Current Concepts In Cartilage Repair
Given the challenges of addressing lower extremity cartilage injuries, this author offers a thorough review of the literature on microfracture repair, osteochondral allograft transfer system (OATS) procedures and autologous chondrocyte implantation, and assesses the potential of newer allogeneic materials.
 Injury to articular cartilage remains a troublesome condition that often produces chronic pain and dysfunction for the patient. This kind of injury challenges the treating physician in restoring a pain-free, functional joint. There are many factors that contribute to this difficulty but most are intrinsic to the native tissue and its healing capability.
Injury to articular cartilage remains a troublesome condition that often produces chronic pain and dysfunction for the patient. This kind of injury challenges the treating physician in restoring a pain-free, functional joint. There are many factors that contribute to this difficulty but most are intrinsic to the native tissue and its healing capability.
By its very nature, cartilage is avascular, acellular and not innervated. Its lack of vascular supply limits an inflammatory response to injury necessary for healing. The limited chondrocytes that are present are packed into a thick extracellular matrix, which makes migration difficult to the site of the injury. The fact that this tissue does not demonstrate innervation brings into question the cause of the pain for our patients.
Functionally, cartilage is designed to transmit forces across a diarthrodial joint. This functionality adds to the challenge in its restoration as articular cartilage has little tolerance for anomaly. Structurally, articular cartilage has well defined zones of hierarchy that are often difficult to imitate during cartilage restoration. The deep zone intimate with the subchondral plate has vertically oriented collagen fibers. These fibers are analogous to Sharpey’s fibers that connect periosteum to bone but in this case, the collagen fibers in this deep zone connect cartilage to the subchondral bone. The middle zone demonstrates larger spherical chondrocytes in extracellular matrix with higher water content, suggestive of this layer’s dissipative function during loading. The superficial zone closest to the joint demonstrates flat, parallel collagen fibers with limited proteoglycans designed to resist tensile forces.
Initially, the thought was that reproducing articular cartilage would be a simple process of combining cellular scaffolds with appropriate growth factors and appropriate mechanical loading. Unfortunately, this has not been the case. In my observation, engineered neocartilage has shown limitations including a lack of necessary layered hierarchy, mechanical mismatch with native cartilage and inadequate adhesion between native tissue and the implanted scaffold.
 The goal of surgical treatment is the restoration of the subchondral plate and articular cartilage similar to that of native cartilage in terms of type II collagen content, proteoglycan content, collagen orientation and contact mechanics while providing the patient long-term symptomatic relief. Surgeries to address cartilage injury can be either reparative or restorative.
The goal of surgical treatment is the restoration of the subchondral plate and articular cartilage similar to that of native cartilage in terms of type II collagen content, proteoglycan content, collagen orientation and contact mechanics while providing the patient long-term symptomatic relief. Surgeries to address cartilage injury can be either reparative or restorative.
What You Should Know About The Microfracture Technique
With reparative techniques, surgeons can employ procedures to harness the body’s ability to heal itself. The most recognized of these procedures is microfracture. With the microfracture procedure, the surgeon drills the intact subchondral plate in a systematic matter to allow for a migration of pluripotent cells to the injured site, differentiation of these cells to chondrocytes and the subsequent production of cartilage-like tissue. Short- and intermediate-term results are quite good with this technique, particularly when it comes to talar dome lesions.
However, there are drawbacks. Fibrocartilage often replaces true hyaline cartilage. Lesion size does influence outcomes, particularly in the talar dome where smaller lesions less than 1 cm2 tend to fare better. Finally, there is limitation of repair sustainability as the fibrocartilage that forms will sooner or later demonstrate fibrillation and ulcerative changes.
To date, there are limited studies discussing the long-term outcome of this procedure with endpoints of greater than 10 years. A recent level IV study by Polat and colleagues reviewed 82 patients who had the microfracture technique for the repair of talar dome lesions over a 13-year period.1 The study had a minimum five-year follow-up with a mean of approximately 10 years. The authors assessed clinical and radiographic outcomes. At the last follow-up, 42 percent of patients had no symptoms and 23 percent of patients had pain after two hours of walking. The authors concluded that microfracture of osteochondral lesions of the talus is a good option with acceptable long-term outcomes in select patients. They also found that the size of the lesion and age of the patient did not impact functional outcome.
 In contrast, Deol and coworkers found in a review of orthopedic literature that contained osteochondral lesions of the talus that were of reduced size in younger patients were independent predictors for surgical success.2 Researchers have also considered the technique in executing this procedure and its potential impact on outcome. In a study by Choi and Lee, the authors looked at arthroscopic subchondral drilling in comparison with microfracture.3 They concluded there was no difference between the two techniques in the stimulation of bone marrow in small to mid-range lesions of the talus with similar outcomes.
In contrast, Deol and coworkers found in a review of orthopedic literature that contained osteochondral lesions of the talus that were of reduced size in younger patients were independent predictors for surgical success.2 Researchers have also considered the technique in executing this procedure and its potential impact on outcome. In a study by Choi and Lee, the authors looked at arthroscopic subchondral drilling in comparison with microfracture.3 They concluded there was no difference between the two techniques in the stimulation of bone marrow in small to mid-range lesions of the talus with similar outcomes.
When considering surgical reparative techniques for these lesions, the surgeon must consider the overall quality of the underlying bone and subchondral plate. Injuries with failed “subflooring” that demonstrate loss of subchondral plate integrity and/or possess cystic degeneration of bone become a new challenge. There must be infrastructure to support the repaired or regenerating cartilage. This is particularly true for larger lesions in which bone grafting techniques or osteochondral grafts may be required.
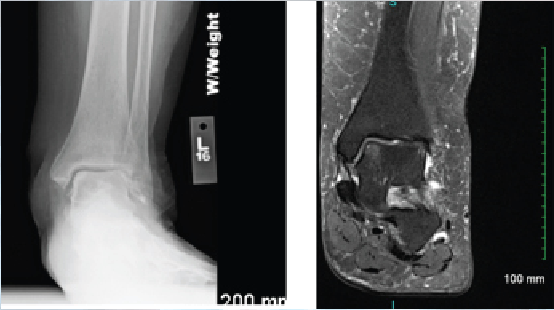
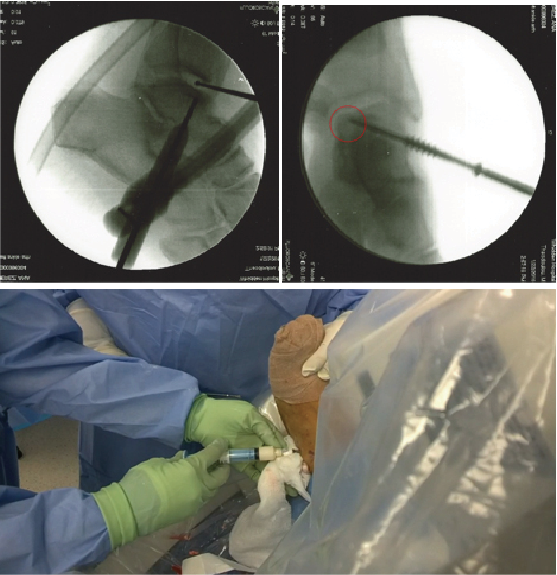
Interestingly, Lee and colleagues looked at the microfracture technique in the presence or absence of subchondral cysts when addressing lesions of the talus.4 They found that microfracture alone produced good outcomes in small to mid-sized lesions regardless of the presence of a cyst. Conversely, the cartilage may remain intact but the underlying flooring has developed cystic changes. In these cases, one may address only the cyst with preservation of the joint surface. Medial talar dome lesions are quite amenable to this through a retrograde technique. In this case presentation, radiographic and magnetic resonance image (MRI) studies suggest intact overlying cartilage with underlying bone marrow edema and early erosive changes.4
In this approach as described by Hyer and colleagues, the authors reviewed eight consecutive patients with a mean follow-up of 24 months.5 They found short-term outcomes comparable to previously described techniques for retrograde drilling of these posteromedial lesions.
 What The Research Says On Osteochondral Autologous/Allograft Transfer Systems
What The Research Says On Osteochondral Autologous/Allograft Transfer Systems
Surgeons have also employed osteochondral allograft transfer systems (OATS) in the management of cartilage injuries. These procedures are helpful when the injury is beyond the cartilage and extends through the subchondral plate, and into underlying bone. They are also useful when the size of the lesion is no longer amenable to bone marrow stimulation techniques.
One commonly obtains autologous grafts from non-loading articular surfaces of other joints. The ipsilateral posterior femoral condyle of the knee is a graft source and, to a less frequent extent, the non-articulating portion of the talar head can be a source. Unfortunately, studies have shown complications of up to 50 percent at the donor site with residual pain.6 Further, researchers have also found that the transplanted articular cartilage does not maintain a hyaline nature but dedifferentiates to a fibrocartilage.6
With regard to functional and MRI outcomes, Flynn and coworkers recently looked at patients treated with an OATS technique.6 Commonly, surgeons have reserved this procedure for lesions of 15 mm in diameter or greater, the presence of subchondral cyst, and/or failed prior microfracture. In this study, the average size of the lesions was smaller at 10 mm in diameter. The authors concluded the procedure provided good outcomes for large lesions of the talus but T2 MRI suggested the graft did not mirror that of native hyaline cartilage. The authors did not comment on the long-term implication of this finding as it is not known.
 In order to limit the impact of morbidity associated with autologous donor sites, researchers have looked at autograft versus allograft and respective outcomes. In one randomized study, Ahmad and Jones looked at 40 consecutive patients with 20 patients receiving autograft plugs from the ipsilateral knee and the other 20 patients using allograft plugs from a size-matched fresh talus.7 They noted similar outcomes between the two groups, suggesting the avoidance of graft site complications in the autologous group.
In order to limit the impact of morbidity associated with autologous donor sites, researchers have looked at autograft versus allograft and respective outcomes. In one randomized study, Ahmad and Jones looked at 40 consecutive patients with 20 patients receiving autograft plugs from the ipsilateral knee and the other 20 patients using allograft plugs from a size-matched fresh talus.7 They noted similar outcomes between the two groups, suggesting the avoidance of graft site complications in the autologous group.
We can divide regenerative surgeries into cellular versus scaffold approaches. Scaffold approaches rely on biomaterials to provide structure and mechanical strength for cartilage regeneration with biocompatible zones. The phenotypic expression of embedded cells relies on biologic and chemical cues. Scaffolds can be either natural or synthetic in makeup. Natural scaffolds can be polymers such as alginate or chitosan. Surgeons have used protein structures such as collagen with these techniques. Vila Y Rico and colleagues suggest the use of a chitosan polymer in combination with bone marrow stimulation.8 The authors hypothesize that applying the bioscaffold allows for a more stable blood clot and provides a three-dimensional structure for the mesenchymal cells to populate.
Key Insights On Autologous Chondrocyte Implantation
Cellular techniques may represent chondrocyte or pluripotent cell application. It requires cell directed expression in appropriate zonal differentiation. Autologous chondrocyte implantation requires two operative procedures to harvest cartilage cells. This is followed by plate expansion and subsequent reintroduction to the injured site. The positive to autologous chondrocyte implantation is that there is little injury to the host harvest site, unlike the OATS procedure, and it is autologous, which limits immunogenicity to the graft.The negatives are that autologous chondrocyte implantation requires two procedures, there is a long recovery time of six to 12 months for maturation of the neocartilage, it is costly and there is suggestion that without a 3D scaffold structure, these cells dedifferentiate back to fibrocartilage.
With that in mind, matrix-assisted autologous chondrocyte implantation is a consideration. Harvesting of cartilage cells begins with initial arthroscopy. One then populates cells onto a type I/III collagen matrix, expands the cells and re-implants them.
 Wylie and colleagues did a systematic review of the matrix effect on cartilage repair.9 They concluded that patient-reported outcomes were better for those whose repair occurred with matrix autologous chondrocyte implantation versus microfracture alone. To avoid a two-step process, there is increasing use of bone marrow aspirate in a one-stage technique of grafting. In this process, one debrides and drills the lesion, and applies concentrated autologous bone marrow aspirate to the area.
Wylie and colleagues did a systematic review of the matrix effect on cartilage repair.9 They concluded that patient-reported outcomes were better for those whose repair occurred with matrix autologous chondrocyte implantation versus microfracture alone. To avoid a two-step process, there is increasing use of bone marrow aspirate in a one-stage technique of grafting. In this process, one debrides and drills the lesion, and applies concentrated autologous bone marrow aspirate to the area.
Hannon and coworkers recently looked at 22 patients who had bone marrow stimulation with concentrated bone marrow aspirate in comparison to 12 patients who simply had bone marrow stimulation.10 The mean follow-up was 48 months. While both procedures showed similar medium-term functional outcomes, those who also had application of concentrated bone marrow aspirate demonstrated improved integration of the repaired tissue with reduced fissuring and fibrillation as visible on MRI, suggesting potentially better long-term results.
A Closer Look At Innovative Allografts
There are newer allogeneic materials available to assist in the repair of cartilage injuries. These include those that are cellularized and decellularized.
BioCartilage (Arthrex) is a dehydrated cartilage allograft represented by morselized cartilage with particle sizes of 100 to 300 microns. It contains type II collagen, proteoglycans and growth factors. BioCartilage serves as a scaffold with potential for autologous cellular signal following microfracture.
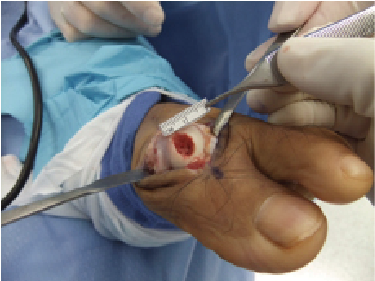
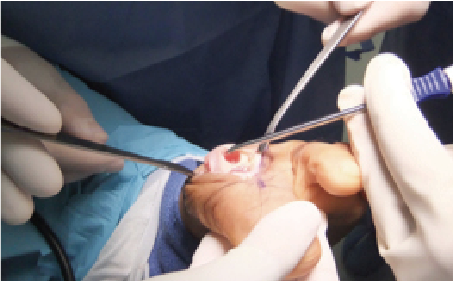
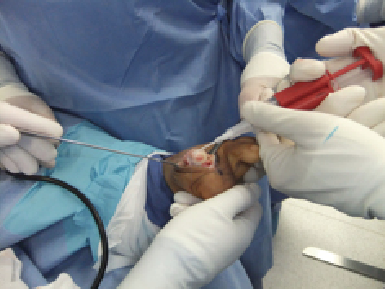
Depicted in the photos above is a case involving the treatment of an osteochondral lesion of the first metatarsal head with BioCartilage. One would appropriately prepare the lesion of the metatarsal head with debridement and drilling. Reconstitute the morselized cartilage allograft with whole blood, platelet rich plasma or concentrated bone marrow aspirate, and apply the allograft. After fully applying the allograft, secure it with a fibrin glue product.
 It is too early to appreciate the benefits of this allograft technique. In an equine model, Fortier and coworkers suggest improved cartilage repair when using BioCartilage in comparison to microfracture alone for full-thickness cartilage loss.11
It is too early to appreciate the benefits of this allograft technique. In an equine model, Fortier and coworkers suggest improved cartilage repair when using BioCartilage in comparison to microfracture alone for full-thickness cartilage loss.11
Juvenile particulated articular cartilage originates from donors under age 13. It has a higher chondrocyte count and increased allogeneic stem cells. Unlike autologous chondrocyte implantation, a singular procedure is required. One would reserve juvenile particulated articular cartilage for cases in which patients have failed non-operative or prior operative intervention, and those cases involving lesions of acceptable size (smaller versus larger). There is higher cost with BioCartilage. At present, there is a lack of studies documenting outcomes using this type of graft.
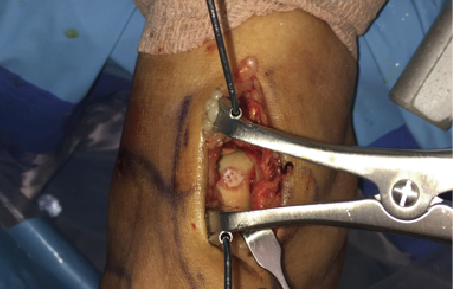
A more recent addition on the market is a cryopreserved viable osteochondral allograft called Cartiform (Osiris Therapeutics/Arthrex). This too is harvested from a juvenile host. It is comprised of extracellular matrix, viable chondrocytes and chondrogenic growth factors. It is a scaffold onto itself and promotes an appropriate structure for phenotypic expression of new cartilage cells. Cartiform provides the benefit of a fresh allograft without the challenges of its use from the standpoint of procurement and infection transmission. One can see the application of such a graft to a lesion on the central medial aspect of the talus (see above left photo).
In Conclusion
Damage to articular cartilage remains a challenging condition to treat and provide sustainable outcomes. The highly specialized nature of this tissue and its inherent lack of self-healing are significant obstacles to overcome. At present, my approach to focal lesions remains addressing for the “here and now” by doing the least to get the most. The patient must understand our current limitations while clinical practice in collaboration with research in bioengineering continue to advance our knowledge and generate more long-lasting outcomes for joint preservation.
Dr. Theodoulou is an Attending Surgeon at Cambridge Health Alliance and an Instructor of Surgery at Harvard Medical School.
References
- Polat G, Ersen A, Erdil M, Kizilkurt T, Kilicoglu O, Asik M. Long-term results of microfracture in the treatment of the talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2016; 24(4):1299-303.
- Deol P, Cuttica D, Smith W, Berlet G. Osteochondal lesions of the talus:size, age and predictors of outcomes. Foot Ankle Clinic. 2013; 18(1):13-34.
- Choi J, Lee K. Comparison of clinical outcomes between arthroscopic subchondral drilling and microfracture of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016; 24(7):2140-7.
- Lee K, Park H, Cho H, Seon J. Comparison of arthroscopic microfracture for osteochondral lesions of the talus with and without subchondral cyst. Am J Sports Med. 2015; 43(8):1951-6.
- Hyer C, Berlet G, Philbin T, Lee T. Retrograde drilling of osteochondral lesions of the talus. Foot Ankle Spec. 2008; 1(4):209-9.
- Flynn S, Ross K, Hannon C, Yasui Y, Newman H, Murawski C, et al. Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2016; 37(4):363-72.
- Ahmad J, Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent of large talar osteochondral lesions. Foot Ankle Int. 2016; 37(1):40-50.
- Vila Y Rico J, Dalmau A, Chaques F, Asuncion J. Treatment of osteochondral lesions of the talus with bone marrow stimulation and chitosan-glycerol phosphate/blood implants. Arthrosc Tech. 2015; 4(6):663-7.
- Wylie J, Hartley M, Kapron A, Aoki S, Maak T. What is the effect of matrices on cartilage repair? A systematic review. Clin Orthop Relat Res. 2015; 473(5):1673-82.
- Hannon C, Ross K, Murawski C, et al. Arthroscopy bone marrow stimulation and concentrated bone marrow aspirate for osteochondral lesions of the talus: a case-controlled study of functional and magnetic resonance observation of cartilage repair tissue outcomes. Arthroscopy. 2016; 32(2):339-47.
- Fortier L, Chapman H, Pownder S, et al. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016; 44(9):2366-74.
For further reading, see “A Closer Look At Drilling And Cartilage Replacement For Talar Dome Lesions” in the February 2015 issue of Podiatry Today or “Emerging Advances In Ankle Cartilage Repair” in the September 2013 issue.











