Combating Biofilms In The Chronic Wound
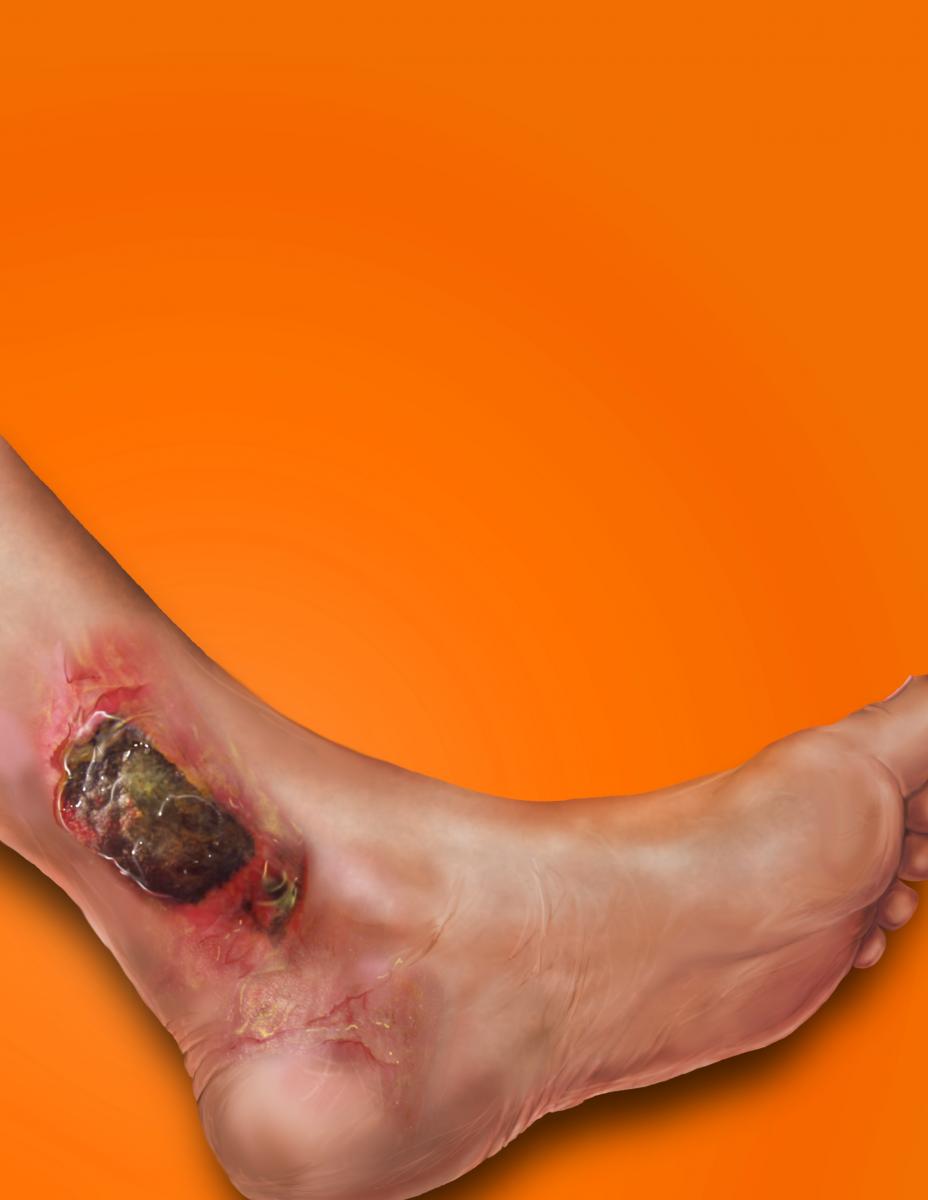 Given the complexity of biofilm in lower extremity wounds, these authors offer a closer look on how biofilm develops, keys to eradicating biofilm and emerging modalities that may have an impact in the future.
Given the complexity of biofilm in lower extremity wounds, these authors offer a closer look on how biofilm develops, keys to eradicating biofilm and emerging modalities that may have an impact in the future.
We all encounter biofilms on a regular basis in our practices. A biofilm is a complex polymicrobal community of bacteria and fungi that develops on foreign materials, necrotic debris, exposed bone, and within the bed of chronic wounds. When James and colleagues examined the biopsies of 50 chronic wound beds, 60 percent contained a biofilm.1
Planktonic or free-floating bacteria are more aggressive and divide more rapidly. Changes in gene expression allow them to secrete hydrolase enzymes and exotoxins, resulting in more rapid local tissue invasion. As a bacterial colony develops, environmental stimuli induce the cells to engage in quorum sensing, a gradient-based recruitment strategy used to summon additional bacteria to the developing biofilm and alter the phenotypic expression of bacteria within the community. Free-floating planktonic bacteria adhere to the wound bed using very weak molecular interactions.
If the immune system does not quickly identify and remove the initial invading organisms, or if the physician does not debride the organisms from the wound bed, they will anchor themselves more permanently and become sessile using cell surface adhesion molecules as well as other cell to cell adhesion molecules. Within the biofilm, bacteria undergo a different phenotypical change, coalesce and begin to secrete a protective extracellular polymeric substance. This substance forms a barrier around the bacterial community, making it very difficult for white blood cells, metalloproteases, antibiotics and even oxygen to penetrate.2 The extracellular polymeric substance secreted around the biofilm makes the community up to 1,000 times more resistant to antiseptics and antibiotics.3
It is for this reason that aggressive, frequent debridement, high doses of antimicrobial agents and prolonged treatment courses are often required to manage an established biofilm effectively. New approaches to infections are not relying solely on developing new antibiotics but using strategies that disrupt the biofilm in various ways, making the organisms more susceptible to current antibiotics.
Current Insights On Bacteria Communication And Biofilm Development
Bacteria communicate with one another by emitting chemical mediators in a process known as quorum sensing. This complex phenomenon enables members of the bacteria’s planktonic cohort to aggregate, form communities and respond to the wound environment.4 When the established chemical gradient reaches a concentration threshold, it triggers changes in gene expression that alter the phenotypic characteristics of the planktonic cohort, allowing them to act more as a solitary unit as opposed to an individual bacterium.
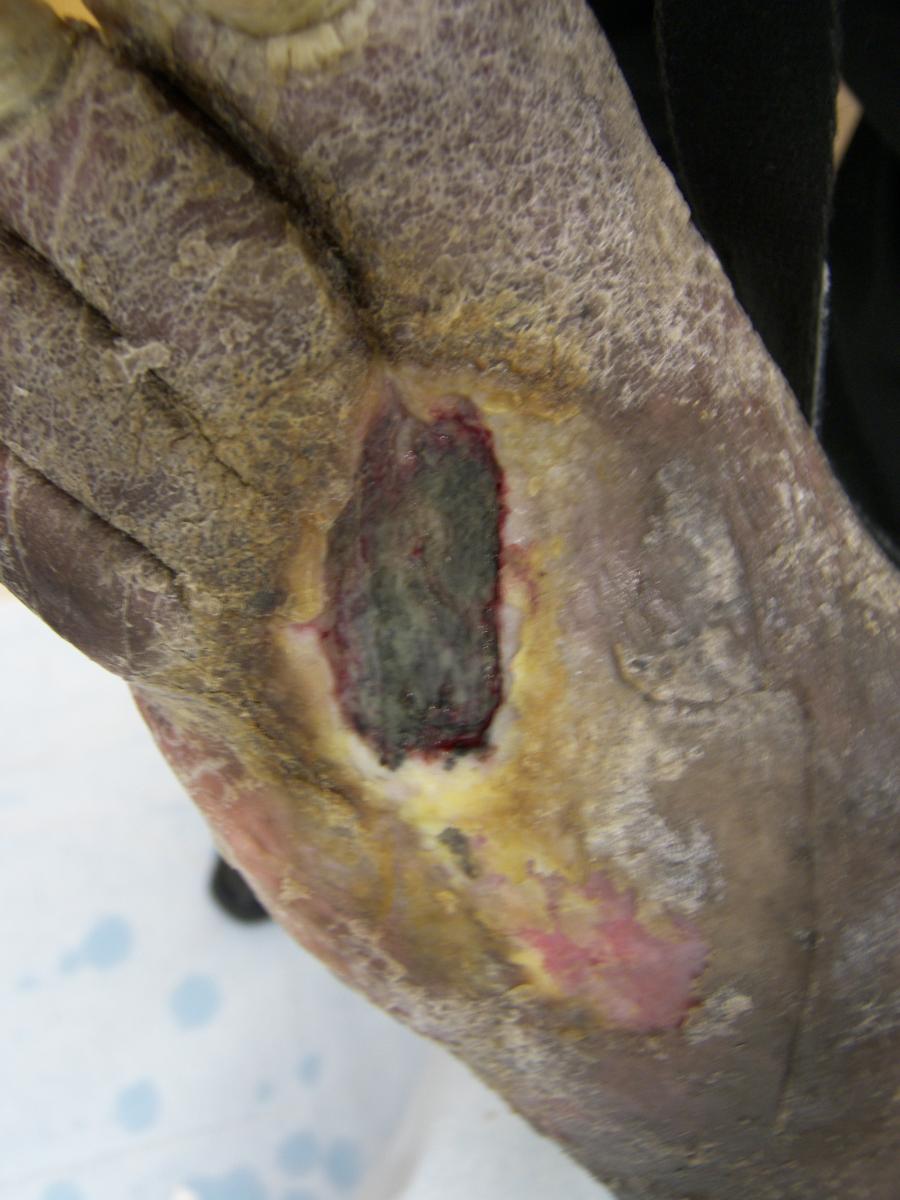 This phase of biofilm development is currently under research as a target for pharmacological intervention. Recent studies on rats have demonstrated that a quorum-sensing inhibitor RNA III-inhibiting peptide was able to eliminate medical device–associated staphylococcal infections.5 This suggests that medical implants coated with an RNA III-inhibiting peptide could be effective at preventing postoperative biofilm formation.
This phase of biofilm development is currently under research as a target for pharmacological intervention. Recent studies on rats have demonstrated that a quorum-sensing inhibitor RNA III-inhibiting peptide was able to eliminate medical device–associated staphylococcal infections.5 This suggests that medical implants coated with an RNA III-inhibiting peptide could be effective at preventing postoperative biofilm formation.
As other species of bacteria enter the community, the true biofilm begins to manifest itself. The community begins to develop zones of activity with more metabolically active forms of the bacteria existing on the surface of the biofilm and inactive anaerobic forms at the base or center of the biofilm. These quiescent cells are what we refer to as persister bacteria. The persister bacteria are sheltered from immune system components and have significantly decreased target sites for antimicrobials so they can withstand extreme environmental conditions and multiple antibiotic challenges.6 The large number of diverse cells in close proximity is highly conducive to intra- and interspecies interactions, allowing for a somewhat unified response of the community to changes in the biofilm environment.7
Bacterial communication does not always take the form of exogenously emitted signaling molecules but sometimes involves the actual physical transfer of intracellular material by interconnecting nanotubes.8 This may account for the development of resistance genes that are characteristic of one species showing up in other species within the biofilm.
As the biofilm grows and thickens, bacteria constituents near the bottom of the biofilm lose access to vital nutrients and growth factors, which results in a slowing of their mitotic rate. It is for this reason that the size of a biofilm and the metabolic rate of its base constituents have an inverse correlation.
Biofilms spread and colonize new surfaces by a process known as dispersal. Microcolonies within the biofilm may break off by means of physical disruption or through a genetic response that involves the production of enzymes that degrade the extracellular polymeric substance and allow for fracturing of the matrix. Microcolonies that have become detached will travel and seed other areas of the host in the direction of fluid flow. This constant shedding allows for an enduring source of drug-resistant bacteria, which promotes bacterial spread. It also acts as a continual challenge to the host immune response ensuring a constant state of inflammation, thereby producing a continual flow of nutrients to the biofilm. Enzymes such as dispersin B and deoxyribonuclease may one day be useful as anti-biofilm agents.9 Biofilms get their nutrients by stimulating a constant flow of exudate across the biofilm surface.
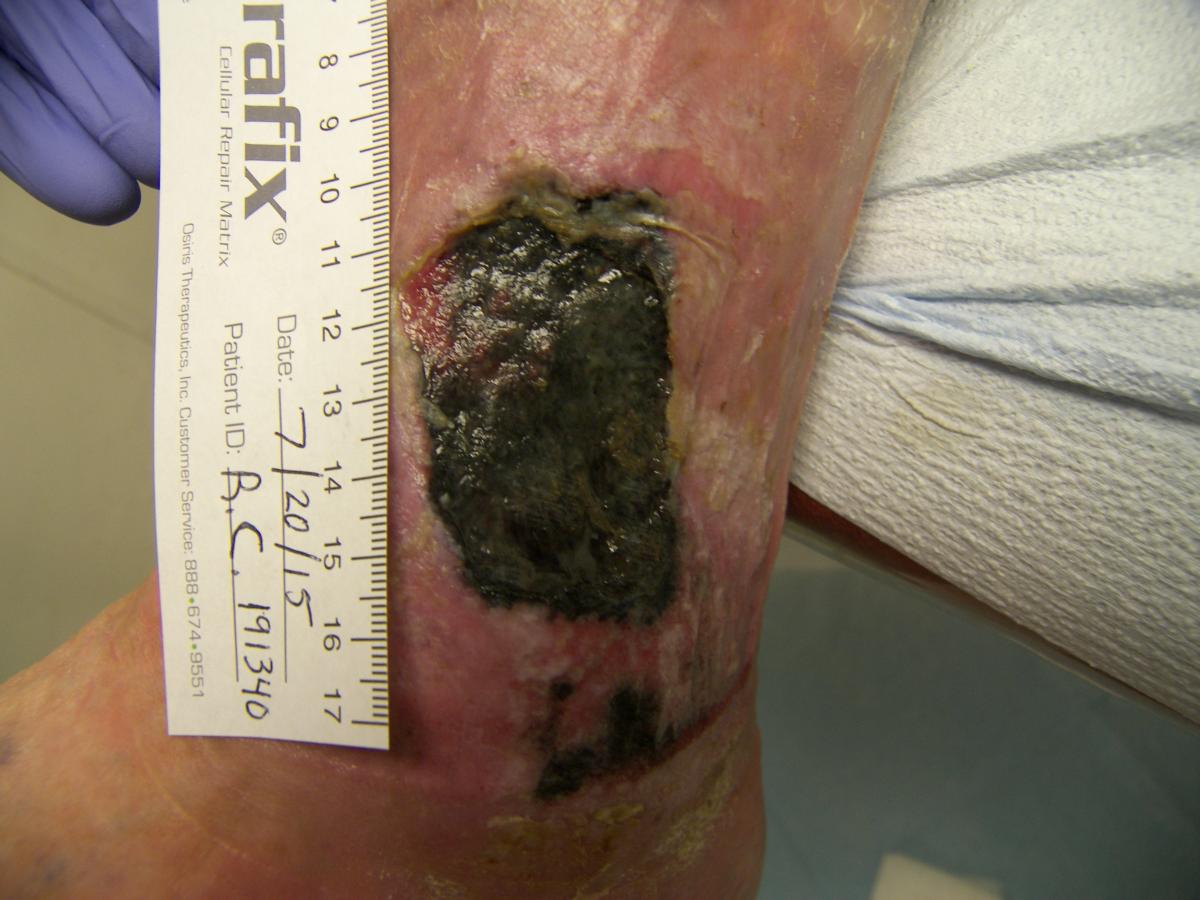 According to Wolcott and colleagues, the objective of a biofilm is not to overwhelm the host but to develop a symbiotic relationship in which the biofilm stimulates an inflammatory response in the host, and the exudate produced in response provides the nutrients the biofilm needs to survive.10 Biofilm communities are geographically unique surfaces consisting of towers, valleys and tunnels of extracellular polymeric substances and bacteria, which allow for the flow of nutrient-rich transudate across and through the community.
According to Wolcott and colleagues, the objective of a biofilm is not to overwhelm the host but to develop a symbiotic relationship in which the biofilm stimulates an inflammatory response in the host, and the exudate produced in response provides the nutrients the biofilm needs to survive.10 Biofilm communities are geographically unique surfaces consisting of towers, valleys and tunnels of extracellular polymeric substances and bacteria, which allow for the flow of nutrient-rich transudate across and through the community.
Assessing The Impact Of Staphylococcus In Biofilms
When utilizing standard culture techniques, Staphylococcus aureus is by far the most commonly identified pathogen in lower extremity biofilms. When one uses polymerase chain reaction (PCR) and DNA sequencing techniques to identify wound pathogens, a much more diverse picture arises. Multispecies biofilms (eight to 16) are much more common than the three to five organisms identified with cell-based culture techniques. Although molecular diagnostics may soon replace cell-based techniques, we have not arrived there quite yet. Fascinating work is occurring to define the normal skin biome as well as the difference between a healthy and pathological wound biome.
Staphylococcus is a normal constituent of the human skin flora and resides in large concentrations in the nostrils. Staphylococcus is able to produce a slime layer, or glycocalyx, that it uses to attach to surfaces. Staphylococcus often adheres to implanted medical devices because upon implementation, the devices get coated in a layer of host proteins. The glycocalyx is able to bind to those proteins to begin forming a biofilm. In addition, Staphylococcus is able to produce a wide variety of virulence factors that it uses to evade the host’s immune defenses. Staphylococcus is also able to upregulate osteoclastic activity, making it particularly adept at developing osteomyelitis.
What You Should Know About Chronic Osteomyelitis As A ‘Bone Biofilm’
The increasing incidence of diabetes has contributed to a rise in osteomyelitis infections in the past 10 years. Waldvogel and coworkers determined that approximately one-third of all patients with osteomyelitis had diabetes.11
Researchers have defined chronic osteomyelitis as a “bone biofilm.”12 The resistance of osteomyelitis to antibiotic therapy is largely attributed to the ability of its causative organisms to form a biofilm on bone surfaces when the infection becomes chronic in nature. Despite recent advances in antibiotic therapies, the incidence of chronic osteomyelitis has steadily risen because of our inability to address the biofilm component of the infection.13 This has led to radical debridement as the preferred method of treatment in comparison to medical management of the disease.
Biofilm-Based Wound Care: Where We Are
What we have learned about bacteria is that they attach within minutes after coming in contact with the wound bed.14,15 Bacteria form attached microcolonies in two to four hours, and form extracellular polymeric substance and develop resistance to therapies in six to 12 hours. Bacteria develop mature biofilms and shed planktonic bacteria in two to four days, and recover from biofilm disruption and reform a biofilm within 24 hours.
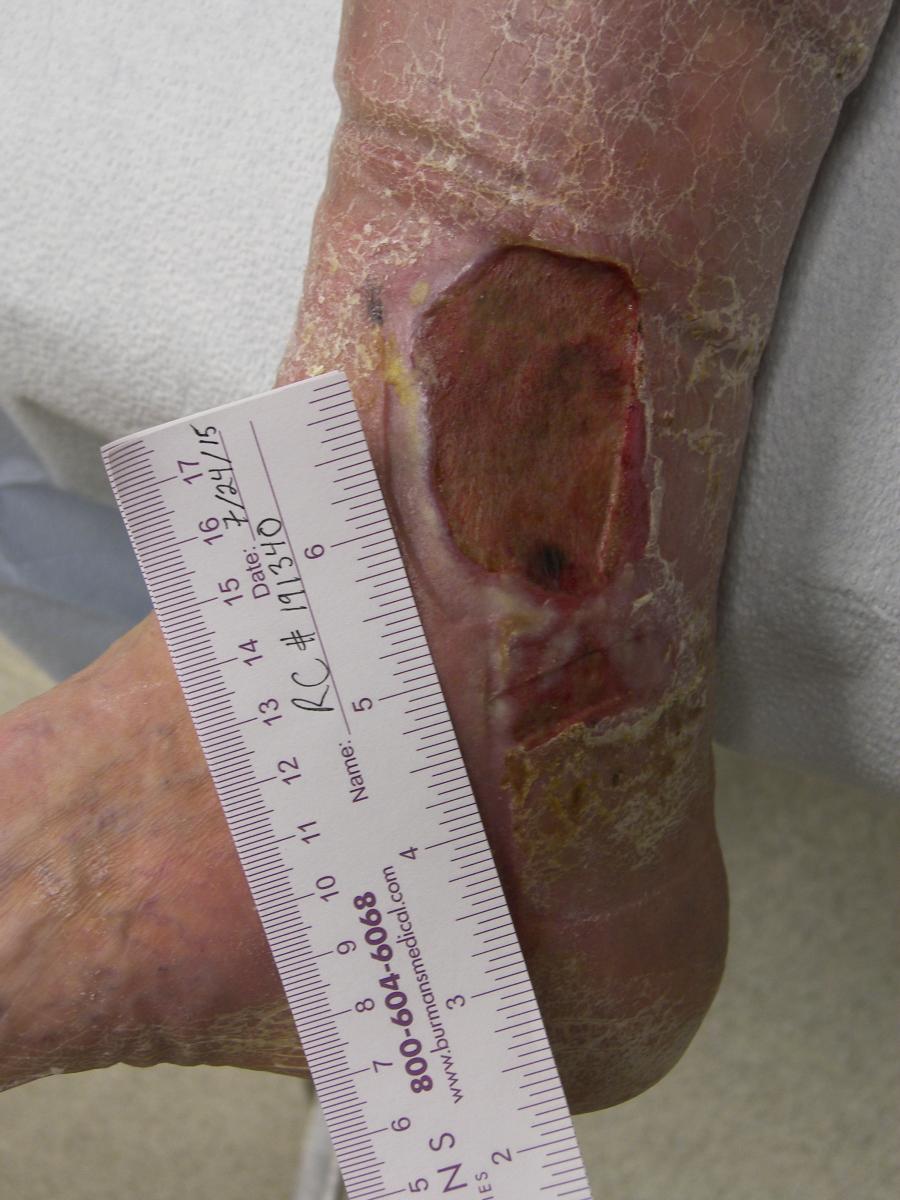 According to Wolcott, there are certain clinical indicators of a biofilm on a chronic wound.16 Suspicion should arise when you see a delay in wound healing despite optimal wound management. Often the wounds appear to have developed a gelatinous material on the wound bed between dressing changes. One can easily remove this material from the wound surface with cleansing or mechanical debridement, but the material reforms quickly after removal. The wound itself will show signs of secondary infection such as excess exudate formation, a change in the wound bed with poor quality granulation tissue, local inflammation, an increase in odor and an increase in pain at the site.
According to Wolcott, there are certain clinical indicators of a biofilm on a chronic wound.16 Suspicion should arise when you see a delay in wound healing despite optimal wound management. Often the wounds appear to have developed a gelatinous material on the wound bed between dressing changes. One can easily remove this material from the wound surface with cleansing or mechanical debridement, but the material reforms quickly after removal. The wound itself will show signs of secondary infection such as excess exudate formation, a change in the wound bed with poor quality granulation tissue, local inflammation, an increase in odor and an increase in pain at the site.
Physicians may also see an antibiotic failure, recurrent infection or signs of infection following completion of antibiotic therapy with negative standard cultures. One should suspect a biofilm when an infection lasts longer than 30 days or when a wound responds positively to systemic or topical steroids that suppress the inflammation being generated by the biofilm itself.16
Devising A Plan To Remove Biofilm
The key to successful biofilm-based wound care is to employ multiple concurrent strategies (via physical, chemical and biological means) to remove, disrupt and prevent recurrence of the infection. When it comes to physical disruption of the biofilm, performing frequent surgical or mechanical debridement such as ultrasonic debridement can remove accumulated biofilm from the wound bed.17
Dressings can physically disrupt the biofilm. These include hydroconductive or high fluid volume dressings that absorb and contain high volumes of wound fluid and bacteria in the dressing itself. Recently, researchers have employed a dressing coated with the fatty acid dialkyl carbamoyl chloride (DACC) as an alternative contact surface for wound bacteria that irreversibly binds to the DACC and not to the wound itself.18 Negative pressure wound therapy (NPWT) has demonstrated the ability to remove large numbers of bacteria from the wound surface. When clinicians apply NPWT early after debridement or with the instill technique, they can prevent biofilm formation on the granular bed between dressing changes.18
Chemical approaches to biofilm-based wound care include the use of wound friendly antiseptics such as hypochlorous acid, polyhexamethylene biguanides, topical cadexomer iodine, silver, methylene blue/gentian violet and iodine-releasing foam dressings. Other topical anti-biofilm agents such as gallium, ethylenediaminetetraacetic acid and bismuth thiols have potential, but have not been developed commercially at this time.
 There has been an emergence of biosurfactants that inhibit microbial adhesion. Without the initial adhesion step, a biofilm would be unable to develop. For example, coating implantable medical devices with silicone significantly decreases S. aureus and E. coli adhesion in vitro and in vivo. Authors are also studying biological molecules to block the cell surface receptors necessary for adhesion to occur. Cusumano and colleagues found that blocking the interactions of adhesins with their target receptors allowed mice to acquire a successful prophylaxis against urinary infections.5 Another modality in this arena is the high concentration compounded antibiotic preparation. These compounded wound gels use DNA culture-targeted, high concentration antibiotics to overwhelm the bacteria present in the wound bed.
There has been an emergence of biosurfactants that inhibit microbial adhesion. Without the initial adhesion step, a biofilm would be unable to develop. For example, coating implantable medical devices with silicone significantly decreases S. aureus and E. coli adhesion in vitro and in vivo. Authors are also studying biological molecules to block the cell surface receptors necessary for adhesion to occur. Cusumano and colleagues found that blocking the interactions of adhesins with their target receptors allowed mice to acquire a successful prophylaxis against urinary infections.5 Another modality in this arena is the high concentration compounded antibiotic preparation. These compounded wound gels use DNA culture-targeted, high concentration antibiotics to overwhelm the bacteria present in the wound bed.
Biological approaches to biofilms include the use of maggot debridement therapy, which researchers have shown to be effective in removing wound biofilms.19 Maggot debridement has become much easier for clinicians to employ with the invention of a bag containment system that prevents the release of maggots into the wound and makes their disposal much easier. The application of bacteria-specific phages has shown some success in wound management but has not gained wide acceptance in clinical application here in the U.S.20
Are There Promising Developments On The Horizon For Addressing Biofilms?
Certain topical applications in development have a direct effect on bacterial cell function. These topical applications include lactoferrin, xylitol, dispersin B and even honey. Researchers have demonstrated that the quorum sensing inhibitor Hamamelis virginiana, extract from the common witch hazel plant, prevents staphylococcal bacteria from attaching and forming biofilms.21
The art of choosing a therapy such as an antibiotic to treat an infection can at times seem arbitrary. Biofilms are almost always made up of complex community groups of bacteria that confer genes of antibiotic resistance to each other. Traditional cultures can give us a limited set of diagnostic information. Bacteria are fastidious and we can only culture 1 percent of all known bacterial species in a lab with standard agar-based techniques.22 The vast majority of bacteria present in the wound go undetected when we rely on standard culture techniques. There is a whole wealth of information within the bacterial genome that is both diagnostic and prognostic.
The advent of next generation genetic technology will allow us to sequence DNA obtained from biofilm bacteria to create a precise inventory of all members of the bacterial cohort as well as any genes of antibiotic resistance they may contain in a fraction of the time and for a minimal cost in comparison to the time it would take to complete all the specialty cultures needed to identify the same organisms using standard laboratory methods. The use of these technologies will help physicians make more informed decisions on which therapies to use, the frequency and the dosages required, and the necessary duration of therapy. It will also delineate which bacteria are pathological and which we may consider “friendly.”
In Summary
The wound biofilm is a structure of immense complexity that one can treat in a variety of ways. The advent of DNA sequencing technologies has allowed us not only to identify the vast colonies of wound bacteria and develop strategies to address them, but to begin to examine the cells of the wound bed and quantify the specific effect of the therapies we employ. Just as the gut has a complex wound biome or resident symbiotic bacterial population, so does the skin and, by default, the wound.
Understanding the difference between the pathological environment present in the entropic or deteriorating wound, and the “friendly” biome present in the healthy wound may open new forms of biofilm-based care that may one day even include the use of probiotic therapy for our wounds. We are on the forefront of exciting times in the healing of wounds and podiatric physicians are leading the way in the research and clinical application of these groundbreaking findings.
Dr. McGuire is the Director of the Leonard Abrams Center for Advanced Wound Healing and an Associate Professor in the Departments of Podiatric Medicine and Biomechanics at the Temple University School of Podiatric Medicine.
Mr. D’Alessandro is a fourth-year podiatric medical student at the Temple University School of Podiatric Medicine.
References
- James G, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008; 16(1):37–44.
- Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001; 45(4):999–1007.
- Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol. 2006;296(2–3):149–161.
- Balaban N, Stoodley P, Fux CA, et al. Prevention of staphylococcal biofilm-associated infections by the quorum sensing inhibitor RIP. Clin Orthop Relat Res. 2005; 437:48–54.
- Cusumano CK, Pinkner JS, Han Z, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med. 2011; 3(109):109–15.
- Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–31.
- Kuramitsu HK, He X, Lux R, et al. Interspecies interactions within oral microbial communities. Microbiol Mol Rev. 2007;71(4):653–670.
- Dubey GV, Ben-Yehuda S. Intercellular nanotubes mediate bacterial communication. Cell. 2011; 144(4):590–600.
- Xavier JB, Picioreanu C, Rani SA, et al. Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix--a modelling study. Microbiology. 2005; 151(Pt 12):3817–32.
- Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. J Wound Care. 2008; 17(8):333-341.
- Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects (first of three parts). N Engl J Med. 1970;282(5):198–206.
- Brady RA, Leid JG, Calhoun JH, et al. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008; 52(1):13-22.
- Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369-79.
- Costerton J. The etiology and persistence of cryptic bacterial infections: a hypothesis. Rev Infect Dis. 1984; 6(Suppl 3):S608-16.
- Bester E, Kroukamp O, Wolfaardt GM, et al. Metabolic differentiation in biofilms as indicated by CO2 production rates. Appl Environ Microbiol. 2010; 76(4):1189-97.
- Wolcott RD. Biofilm based wound care. In: Sheffield PJ, Fife CE. (eds). Wound Care Practice, Second Edition. Best Publishing, North Palm Beach, 2007.
- Ammons MC. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat Antiinfect Drug Discov. 2010; 5(1):10–17.
- Li T, Zhang L, Han L, et al. Early application of negative pressure wound therapy to acute wounds contaminated with Staphylococcus aureus: An effective approach to preventing biofilm formation. Exp Ther Med. 2016; 11(3):769–776.
- Cazander G, van Veen KEB, Bouwman LH, et al. The influence of maggot excretions on PAO1 biofilm formation on different biomaterials. Clin Orthop Relat Res. 2009; 467(2):536–545.
- Harper DR, Parracho HM, Walker J, et al. Bacteriophages and biofilms. Antibiotics. 2014; 3(3):270–284.
- Kiran MD, Adikesavan NV, Cirioni O, et al. Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol Pharmacol. 2008; 73(5):1578–86.
- Wolcott RD, Gontcharova V, Sun Y, Dowd SE. Evaluation of the bacterial diversity among and within individual venous leg ulcers using bacterial tag-encoded FLX and titanium amplicon pyrosequencing and metagenomic approaches. BMC Microbiol. 2009;9:226.











