ADVERTISEMENT
A Closer Look At The Potential Of Placental Membrane Grafts For Chronic Diabetic Foot Ulcerations
The treatment of chronic diabetic foot ulcers is a challenging endeavor. Fortunately, medical advances continue to provide promising new technologies to aid physicians in healing difficult wounds. The properties and composition of placental membranes make them a very powerful tool in wound care as placental membranes address the unique myriad needs of chronic diabetic foot ulcers (DFUs).
According to the American Diabetes Association, over 29 million Americans have type 2 diabetes, a number that represents over 9 percent of the population.1 Diabetic foot ulcers are the most common complication of diabetes with an annual incidence of 1 to 4 percent and a lifetime risk of 15 to 25 percent.2-4 The annual cost for treating DFUs is an estimated $9 to $13 billion.5
Diabetic foot ulcers are often resistant to wound therapies due to the complicated diabetic wound environment that is characterized by hyperglycemia, hypoxia and high levels of proteases, bacterial antigens, reactive oxygen species and inflammatory cytokines.6
A Primer On The Healing Properties Of The Placenta
 Placental membranes have a long history of use in wound healing dating back more than 100 years ago.7,8 What follows is a basic review and current understanding of placental membrane graft technology.
Placental membranes have a long history of use in wound healing dating back more than 100 years ago.7,8 What follows is a basic review and current understanding of placental membrane graft technology.
Fresh placental tissue has all the components required to support wound healing: a collagen-rich matrix, a cocktail of growth factors and cytokines, and viable endogenous cells including mesenchymal stem cells. Accumulated data point to the importance of mesenchymal stem cells for wound healing and often patients with chronic wounds have a deficiency in mesenchymal stem cells both in number and functionality.9,10
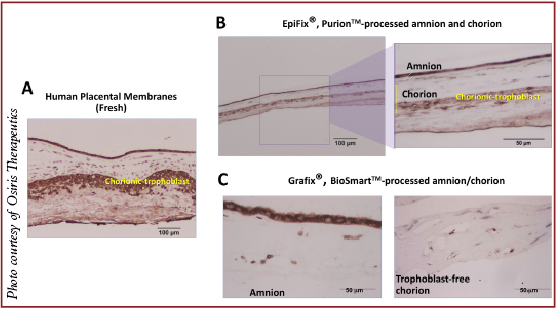
There are two placental membranes: the amnion and the chorion. Historically, due to ease of separation and purification, amnion has been predominant. The literature thoroughly describes the antibacterial, anti-inflammatory and anti-scarring activities of amnion.10
Amnion has two cellular layers: epithelial and stromal (also known as fibroblast, mesenchymal or reticular). The stromal layer contains neonatal fibroblasts and mesenchymal stem cells.
Chorion also has two cellular layers: stromal and trophoblast. The stromal layer of chorion is identical to the stromal layer of the amnion as both have neonatal fibroblasts and mesenchymal stem cells. The trophoblast layer of the chorion contains trophoblasts. This layer is attached to the maternal deciduas and its main function is nutrient exchange between the baby and the mother.
The trophoblast layer within the chorion, at term, contains high levels of inflammatory cytokines and proteases. Physiologically, in utero, as a part of childbirth, these inflammatory cytokines and MMPs are responsible for extracellular placental matrix degradation with the purpose of allowing detachment of the placenta from the maternal uterus.11,12
As we know, chronic wounds stall in the inflammatory phase of wound healing. Accordingly, when considering advanced wound care products, it is important to use those with anti-inflammatory properties. The wound microenvironment is characterized by high levels of inflammatory cytokines such as tumor necrosis factor alpha (TNF-a); matrix metalloproteinases (MMPs), which degrade extracellular matrix and growth factors; and reactive oxygen species. An excess of these factors creates a harsh wound environment, which causes damage to host cells and prevents wounds from proceeding to the proliferative phase of wound healing.13-15 Given this knowledge, wound care products should not contain trophoblasts due to their high levels of MMPs and inflammatory cytokines.
The selection of tissue sources for wound care products matters and yes, there are commercial placental products on the market that contain trophoblasts. EpiFix (MiMedx) is one example whereas others like Grafix (Osiris Therapeutics) do not contain trophoblasts. Due to the trophoblast layer, EpiFix contains high levels of MMPs in comparison to Grafix.
Understanding The Different Commercial Processing Techniques For Amniotic Membrane Grafts
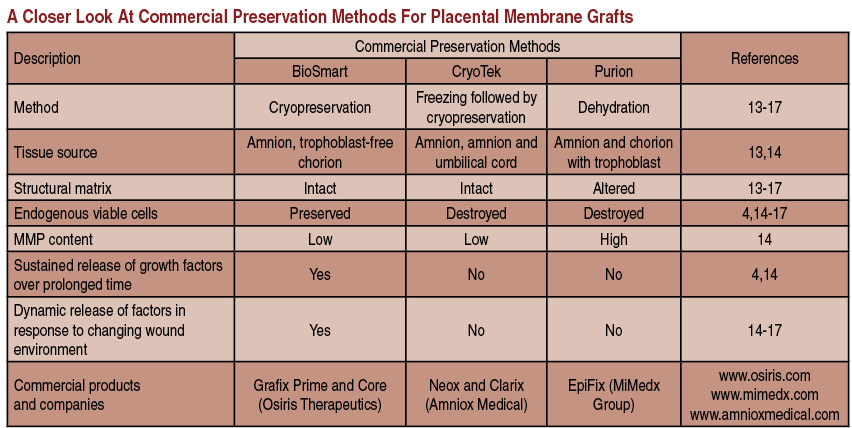
Fresh placental membranes would be optimal to use for wound treatment but their use is difficult due to short storage times. Therefore, different tissue processing methods have emerged. The main goal of tissue processing and preservation is to retain all beneficial components of the tissue as close as possible to the fresh tissue, and achieve a long shelf life.
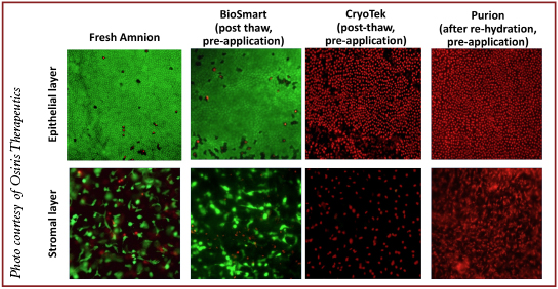
The most popular commercial method of tissue preservation is dehydration with terminal sterilization by radiation, also referred to as Purion™ processing (“dried” amnion or an amnion and chorion combination). The advantage of this method is storage at room temperature. However, dehydration alters the structure of the placental matrix and kills endogenous living cells. The thickness of the placental matrix after dehydration is two- to threefold less in comparison to fresh placental matrix. Examples of dehydrated commercial placental products include: EpiFix and Revitalon (Medline Industries) (amnion and chorion), AmnioExcel (Derma Sciences) and AmnioClear (Liventa Biosciences) (amnion only).16,17
 Another processing method is cryopreservation in glycerol with a freezing step, also known as CryoTek™. This method preserves structural matrix but also kills endogenous living cells. Examples include the devitalized cryopreserved amnion of Neox (Amniox Medical) and Clarix (Amniox Medical), and the devitalized cryopreserved amnion and umbilical cord of Neox Cord and Clarix Cord.18,19
Another processing method is cryopreservation in glycerol with a freezing step, also known as CryoTek™. This method preserves structural matrix but also kills endogenous living cells. Examples include the devitalized cryopreserved amnion of Neox (Amniox Medical) and Clarix (Amniox Medical), and the devitalized cryopreserved amnion and umbilical cord of Neox Cord and Clarix Cord.18,19
At the present time, there are only two cryopreserved placental membrane products on the market with viable endogenous cells. They undergo a BioSmart™ process, which preserves endogenous living cells. Those two grafts are Grafix Prime (amnion) and Grafix Core (trophoblast-free chorion). All components of these placental membranes remain in their native state. Therefore, these products are a true alternative to fresh placental tissues.20
Scientific in vitro data demonstrate the importance of preservation of all components in placental tissues including endogenous living cells. Viable placental tissue has significantly higher magnitude of the effects (anti-inflammatory, antioxidant, chemoattractive and angiogenic) relevant to wound healing. Data indicate that different processing methods have different impacts on placental tissue integrity. Drying causes alterations in structural matrix and kills viable cells. Freezing followed by cryopreservation minimizes matrix alterations but kills viable cells. Finally, controlled rate cryopreservation retains both matrices and keeps viable cells intact. Better preservation of tissue integrity correlates with higher biological activity.21-23
Only viable placental tissues provide sustained release of growth factors in response to a changing microenvironment. Killing endogenous viable cells in placental membranes completely loses this property of fresh tissue in devitalized commercial products.22,23
What You Should Know About Mesenchymal Stem Cells
Mesenchymal stem cells are self-renewing multipotent stem cells that can differentiate into various lineages of mesenchymal origin such as bone, cartilage, tendon and fat.10 Mesencyhmal stem cells are immune privileged, allowing for allogeneic use.24,25 Furthermore, these cells regulate immune response and inflammation, and possess powerful tissue protective and reparative mechanisms, making these cells attractive for tissue repair and regeneration through all the phases of wound healing.
 Chronic wounds typically fail to progress through the normal stages of wound healing (i.e. inflammatory stage followed by proliferative stage and subsequent remodeling.) A significant component of the mechanism of action of mesenchymal stem cells is that they directly attenuate the inflammatory response. Studies have shown that the addition of mesenchymal stem cells leads to a decrease in secretion of TNF-a and interferon gamma (IFN-g) (pro-inflammatory factors) while simultaneously increasing the production of anti-inflammatory cytokines IL-10 and IL-4.26
Chronic wounds typically fail to progress through the normal stages of wound healing (i.e. inflammatory stage followed by proliferative stage and subsequent remodeling.) A significant component of the mechanism of action of mesenchymal stem cells is that they directly attenuate the inflammatory response. Studies have shown that the addition of mesenchymal stem cells leads to a decrease in secretion of TNF-a and interferon gamma (IFN-g) (pro-inflammatory factors) while simultaneously increasing the production of anti-inflammatory cytokines IL-10 and IL-4.26
Researchers have also recognized that mesenchymal stem cells have antimicrobial activity via two mechanisms: directly via secretion of antimicrobial factors such as LL-37 and indirectly via secretion of immune modulating factors, which upregulate bacterial killing by neutrophils and macrophages, and phagocytosis by immune cells.27,28 Mesenchymal stem cell differentiation contributes to the regeneration of damaged tissue whereas mesenchymal stem cell paracrine signaling regulates the local cellular responses to injury. Current data suggests that the paracrine signaling is likely the primary mechanism for the beneficial effects of mesenchymal stem cells on wounds in that it reduces inflammation, promotes angiogenesis and induces cell migration and proliferation.29 Additionally, researchers have demonstrated that the mesenchymal stem cell-conditioned medium promotes dermal fibroblasts to accelerate wound closure.30 In vivo studies have also shown the advantages of using exogenous mesenchymal stem cells for the treatment of wounds.31,32
What The Studies Reveal On Amniotic Membrane Grafts And DFUs
Practicing evidence-based medicine is paramount when treating DFUs. Brantley and Verla recently published an article that concisely reviewed the various studies and trials to date on commercial placental membrane products.6 There are a multitude of case studies available but more substantial studies have also emerged. Prospective DFU trials for commercial placental membranes are currently limited to five studies: one open-label pilot study for Biovance (Alliqua BioMedical); three randomized, controlled trials (two single center and one multicenter) for EpiFix; and one multicenter blinded randomized control trial for Grafix.
There have been randomized, controlled trials but many have been limited by a small population size or variations between the control arm of a study and the treatment arm. A multicenter, blinded RCT shows significantly higher and faster wound closure rates for Grafix with fewer wound-related infections versus standard of care.33 A total of 97 patients from 20 centers across the nation were in the trial: 50 patients with DFUs had weekly applications of Grafix and 47 DFU patients received standard care. Sixty-two percent of Grafix patients closed their wounds versus 21 percent in the standard care group by week 12.
 One large study of note was a retrospective single-center study that utilized Grafix.34 The analysis included 66 patients with 67 wounds, including 27 patients with chronic DFUs. The patients with DFUs had a mean wound size of 3.97 cm2 and a mean wound duration of 24.5 weeks. Twenty-three of the 27 patients with DFUs had previously failed different types of advanced therapies, including collagen matrices, cellular skin substitutes, topical growth factors and negative pressure wound therapy. After 12 weeks of treatment with Grafix, 85.2 percent of the patients with DFUs achieved complete wound closure.
One large study of note was a retrospective single-center study that utilized Grafix.34 The analysis included 66 patients with 67 wounds, including 27 patients with chronic DFUs. The patients with DFUs had a mean wound size of 3.97 cm2 and a mean wound duration of 24.5 weeks. Twenty-three of the 27 patients with DFUs had previously failed different types of advanced therapies, including collagen matrices, cellular skin substitutes, topical growth factors and negative pressure wound therapy. After 12 weeks of treatment with Grafix, 85.2 percent of the patients with DFUs achieved complete wound closure.
In regard to EpiFix, a prospective, randomized, controlled, parallel group, multicenter trial compared the healing effectiveness of treating chronic lower extremity diabetic ulcers with either weekly applications of Apligraf (Organogenesis), EpiFix or standard wound care with a collagen-alginate dressing in a total of 60 patients.35 In the EpiFix group, patients achieved complete wound closure within four and six weeks at rates of 85 and 95 percent respectively in comparison to the rates for Apligraf patients (35 and 45 percent), and the rates for those treated with standard care (30 and 35 percent).
However, a recently published comparison of Apligraf and EpiFix for DFU treatment in a larger patient population shows that the proportion of wounds closed by week 12 was only 28 percent for EpiFix versus 48 percent for Apligraf.36
In Summary
With science and technology continuing to progress at a rapid pace, we encounter new products on a regular basis. It is important to be educated regarding the nuances of these products and to have a firm knowledge of the basic underlying principles and science on which the technologies are founded. Based on this literature review, there are several key questions that one should ask before deciding on a specific placental membrane product to utilize.
• What layers of the placenta does the graft include?
• What processing technique was used and how does it affect placental tissue (matrix, growth factors and viable cells)?
• Are viable cells present in the graft?
• What kind of tests were performed to ensure quality and comparability of a product derived from different placentas? n
Dr. Abshier is in private practice at multiple offices and also works at a wound care center in Ohio.
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimate of Diabetes and Its burden in the United States, U.S. Department of Health and Human Services, Atlanta, 2014.
- Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006; 45(5Suppl):S1-S66.
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719-1724.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. J Am Med Assoc. 2005;293(2):217-228.
- Rice JB, Desai U, Cummings AK, et al. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651-658.
- Brantley JN, Verla T. Use of placental membranes for the treatment of chronic diabetic foot ulcers. Adv Wound Care. 2015; 4(9):545-558.
- Kesting MR, Loeffelbein DJ, Steinstraesser L, et al. Cryopreserved human amniotic membrane for soft tissue repair in rats. Ann Plast Surg. 2008;60(6):684-691.
- Kesting MR, Wolff KD, Hohlweg-Majert B, et al. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29(6):907-916.
- Rodrigues-Menocal L, Salgado M, Ford D, Van Badiavas E. Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med. 2012;1(3):221-229.
- Maxson S, Lopez EA, Yoo D, et al. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1(2):142-149.
- Young A, Thomson AJ, Ledingham M, et al. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002; 66(2):445-449.
- Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002; 87(3):1353-1361.
- Gibson DJ, Schultz GS. Molecular wound assessments: matrix metalloproteinases. Adv Wound Care. 2013; 2(1):18-23.
- Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Rep Regen. 1999;7(6):442-452
- Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermnatol. 2008; 158(5):951-961.
- Kassem RR, Abdel-Hamid AA, Khodeir MM. Effect of lyophilized amniotic membrane on the development of adhesions and fibrosis after extraocular muscle surgery in rabbits. Current Eye Res. 2011; 36(11):1020-1027.
- Kassem RR, Khodeir MM, Salem M, et al. Effect of cryopreserved amniotic membrane on the development of adhesions and fibrosis after extraocular muscle surgery in rabbits. Acta Ophthalmol. 2013; 91(2):e140-e148.
- Thomasen H, Paulkin M, Steuhl KP, Meller D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefes Arch Clin Exp Ophthalmol. 2009; 247(12):1691-1700.
- Cooke M, Tan EK, Mandrycky C, et al. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014; 23(10):465-476.
- Gyurdieva A, Danilkovitch A, Johnson A, Arnold Y. Understanding placental products: impact of commercial preservation methods on anti-inflammatory and angiogenic properties. Symposium on Advanced Wound Care Spring 2015 poster #LB-026 and oral presentation. Presented April 29-May 3, San Antonio, TX.
- Duan-Arnold Y, Gyurdieva A, Johnson A, et al. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care. 2015; 4(6):329-338.
- Duan-Arnold Y, Gyurdieva A, Johnson A, et al. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv Wound Care. 2015; 4(9):523-33.
- Duan-Arnold Y, Gyurdieva A, Johnson A, et al. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care. 2015; 4(6):329-38.
- Banas RA, Trumpower C, Bentlejewski C, et al. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 2008; 69(6):321-328.
- Magatti M, De Munari S, Vertua E, et al. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26(1):182-192.
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105(4):1815-1822.
- Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229-2238.
- Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047-1057.
- Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204-1219.
- Smith An, Willis E, Chan VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316(1):48-54.
- Nakagawa H, Akita S, Fukui M, et al. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153(1):29-36.
- Shumakov VI, Onishchenko NA, Rasulov MF, et al. Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull Exp Biol Med. 2003;136(2):192-195.
- Lavery LA, Fulmer J, Shebekta KA, et al. The efficacy and safety of Grafix for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded clinical trial. Int Wound J. 2014; 11(5):554-60.
- Regulski M, Jacobstein DA, Petranto RD, et al. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage. 2013;59(12):38–43.
- Zelen CM, Gould L, Serena TE, et al. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2014; epub Nov. 26.
- Kirsner RS, Sabolinski MK, Parsons NB, Skornicki M, Marston WA. Comparative effectiveness of a bioengineered living cell construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Rep Regen. 2015;23(5):737-744.
Editor’s note: For related articles, visit the archives at www.podiatrytoday.com .











