Can Carbon Dioxide Angiography Have An Impact For Patients With PAD And Renal Insufficiency?
Angiography with carbon dioxide can be a valuable tool to diagnose and treat patients with peripheral arterial disease and renal insufficiency. These authors take a closer look at the research and practical considerations with CO2 angiography.
A vascular evaluation is one of the most important components of any podiatric physical examination. Diminished pedal pulses, absent hair growth, subjective claudication and/or the presence of chronic wounds and tissue loss might be the first indications that an advanced arterial workup consisting of non-invasive vascular testing is warranted.
Vascular surgeons or interventional cardiologists may then elect to perform an angiogram, which is most commonly completed with an iodinated contrast medium. While other advanced imaging modalities such as magnetic resonance angiography (MRA), computed tomography angiography (CTA) and arterial duplex ultrasound may provide information as to the location of stenotic and occlusive lesions, only angiography allows for the possibility of both diagnosis and intervention in the form of balloon angioplasty and arterial stenting.1
However, the use of this contrast medium might be contraindicated in patients with specific allergies to iodinated contrast or those additionally suffering from chronic kidney disease. This likely represents a common concern. In a study of German patients hospitalized with critical limb ischemia (CLI), 39 percent also had diabetes mellitus and 37 percent had renal insufficiency, defined as a glomerular filtration rate of less than 60 mg/dL.2 Peripheral arterial disease (PAD) affects approximately 12 million people in the United States under the age of 55.3,4 Over 10 percent of this population has concomitant renal insufficiency.3,4
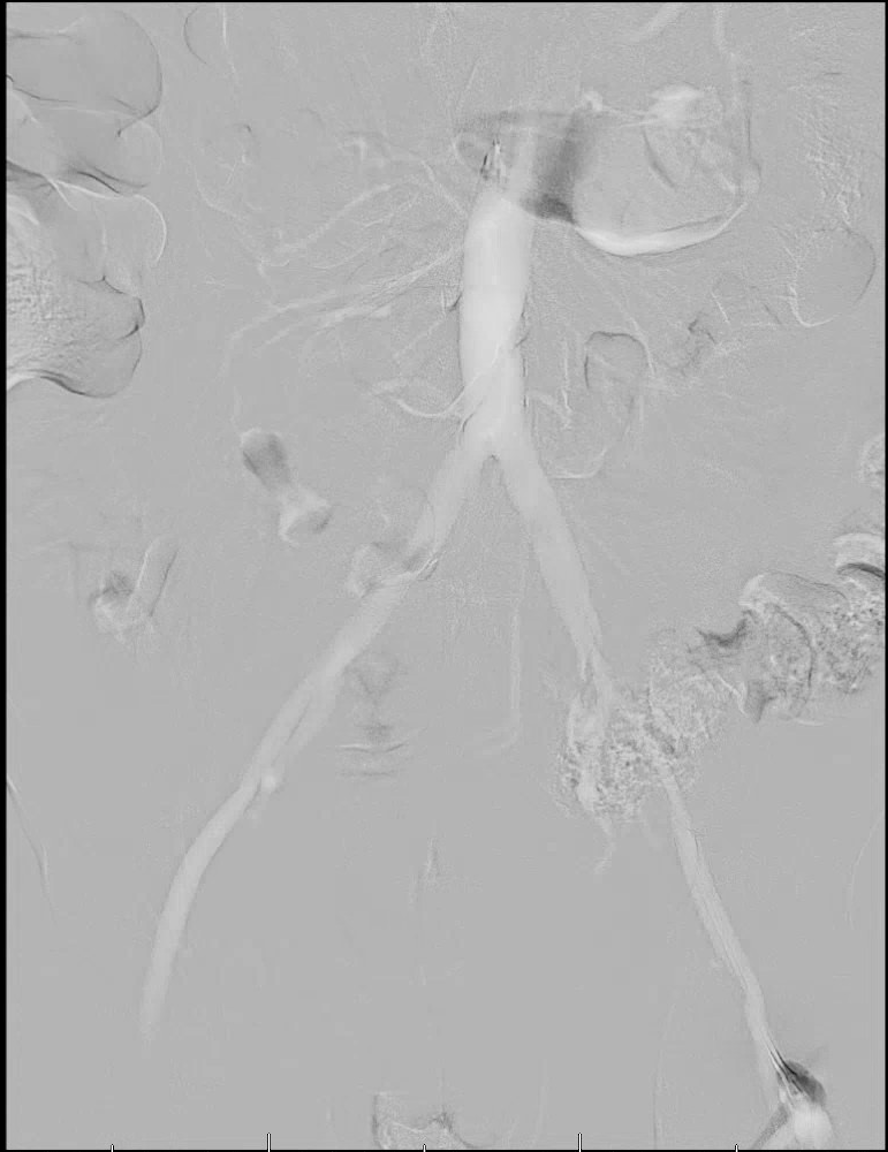 Imaging of the lower extremity arterial supply can be difficult in these situations as diabetic vascular disease commonly affects the smaller infrapopliteal vessels and lesions are usually relatively long (>10 cm) and multisegmental.5 The primary concern with the use of iodinated contrast in these patients is with respect to nephrotoxicity.6 Researchers have defined contrast-induced nephropathy as a post-procedure increase in serum creatinine of 0.5 mg/dL or more than 25 percent from baseline.7 Although we do not fully understand the mechanism of renal injury, it is likely the result of decreased blood flow to the kidneys or contrast cytotoxicity.7 It also appears that the severity of preexisting kidney disease may increase the risk of sustaining further injury to the kidneys. One study found that while only 10.4 percent of patients with a creatinine between 1.3 to 1.9 mg/dL saw an elevation in creatinine following an angiogram, 62 percent of patients with a creatinine greater than 2.0 mg/dL did.8
Imaging of the lower extremity arterial supply can be difficult in these situations as diabetic vascular disease commonly affects the smaller infrapopliteal vessels and lesions are usually relatively long (>10 cm) and multisegmental.5 The primary concern with the use of iodinated contrast in these patients is with respect to nephrotoxicity.6 Researchers have defined contrast-induced nephropathy as a post-procedure increase in serum creatinine of 0.5 mg/dL or more than 25 percent from baseline.7 Although we do not fully understand the mechanism of renal injury, it is likely the result of decreased blood flow to the kidneys or contrast cytotoxicity.7 It also appears that the severity of preexisting kidney disease may increase the risk of sustaining further injury to the kidneys. One study found that while only 10.4 percent of patients with a creatinine between 1.3 to 1.9 mg/dL saw an elevation in creatinine following an angiogram, 62 percent of patients with a creatinine greater than 2.0 mg/dL did.8
This potential nephrotoxicity with iodinated contrast is difficult to avoid. There is some low level evidence to support the use of statins or N-acetylcysteine prior to angiography in order to reduce the risk of kidney injury, and most physicians would agree on the beneficial role of perioperative hydration.7,9
However, carbon dioxide angiography provides a different option for patients with PAD, diabetes mellitus and either a contrast allergy or chronic kidney disease.
Physicians first used carbon dioxide to visualize retroperitoneal structures in the 1920s.10 In 1971, at the University of Florida, Hawkins “inadvertently injected 70 cm3 of room air into a celiac artery” and was surprised with the visualization it provided.11,12 In 1990, Weaver released a pilot study showing that percutaneous transluminal angioplasty was feasible with the use of carbon dioxide digital subtraction angiography.13 Since then, angiography’s role has expanded to include both diagnostic and interventional procedures of arterial and venous structures including angiography with angioplasties.9,10
How Carbon Dioxide Angiography Works
There are several unique properties of carbon dioxide that allow for its use as a contrast agent.10-12 For starters, carbon dioxide displaces the blood rather than mixing with it like an iodinated contrast. It is also less dense than surrounding bodily tissues and is therefore considered a negative contrast agent. Due to this, digital subtraction fluoroscopy is necessary to remove the surrounding anatomic structures and highlight the vascular structures while performing this technique. The vessels will appear bright on carbon dioxide angiograms whereas they appear dark using traditional methods. The gas is also buoyant, meaning it preferentially fills the anterior portion of larger blood vessels when patients are in a supine position. To improve visualization, physicians often take the feet out of the dependent position and elevate them by about 15 degrees.
Carbon dioxide also has a low viscosity and is 400 times less resistant to flow than contrast medium.10-12 Due to its low viscosity, the gas remains undiluted following injection and is useful in visualizing collateral vessels. Carbon dioxide is 28 times more soluble than oxygen and thus does not usually form problematic gas emboli when one uses the proper technique. Any carbon dioxide that does reach the lungs is usually eliminated in a single pass.
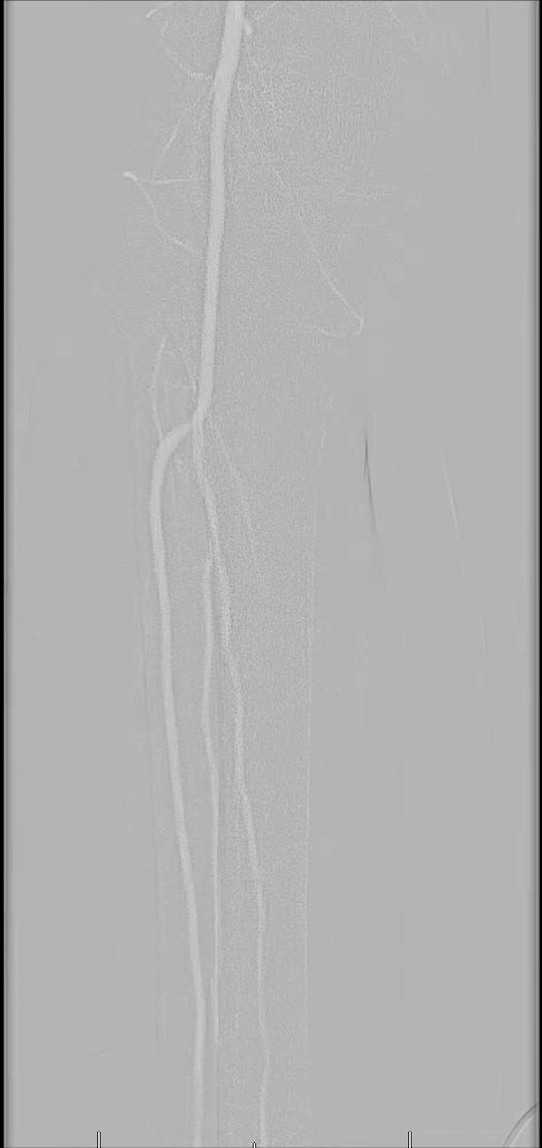 One injects the carbon dioxide either manually or with an automated injector system.10-12 For the distal arteries like the leg, ankle and foot, inject a quantity of 15 to 20 mL of carbon dioxide over a span of two to three seconds. This rate is slightly faster than the flow of blood in that area. If the injection is too slow, then the vessel will not fill completely and if the injection is too fast, the gas may reflux proximal to the injection site and cause pain. In order to allow the lungs to eliminate the gas properly, several minutes should pass between injections and one should avoid excessive gas volumes.
One injects the carbon dioxide either manually or with an automated injector system.10-12 For the distal arteries like the leg, ankle and foot, inject a quantity of 15 to 20 mL of carbon dioxide over a span of two to three seconds. This rate is slightly faster than the flow of blood in that area. If the injection is too slow, then the vessel will not fill completely and if the injection is too fast, the gas may reflux proximal to the injection site and cause pain. In order to allow the lungs to eliminate the gas properly, several minutes should pass between injections and one should avoid excessive gas volumes.
A Closer Look At The Complications And Disadvantages With Carbon Dioxide Angiography
If one injects too large of a bolus or insufficient time passes between injections, a “vapor lock” phenomenon may occur.14,15 This is when a carbon dioxide bubble blocks the pulmonary artery and prevents blood flow, cardiac output and oxygenation. This phenomenon can be worse in patients with chronic obstructive pulmonary disease (COPD) or pulmonary hypertension so allow a greater time between injections in this patient population.10
Do not use carbon dioxide in the thoracic aorta, coronary arteries or cerebral circulation. Animal models have shown local embolism and ischemia of the brain and heart, resulting in neurotoxicity and cardiac arrhythmias, when carbon dioxide is utilized here.10 Rundback additionally reported a case of mesenteric ischemia following a renal angiogram.16 There is also a risk of spinal artery ischemia if the angiogram occurs with the patient in a prone position due to the tendency of the gas to fill nondependent vessels.10,17
Avoid the use of nitrous oxide in patients undergoing carbon dioxide angiography. The nitrogen gas can diffuse into the carbon dioxide bubble and cause it to increase in size, amplifying the risk of vapor lock and ischemia. Another rare complication, which has diminished with improvements in delivery systems, is the possibility of air contamination.18 Close monitoring of the patient’s vitals as well as capnography (measurement of respiratory carbon dioxide) allows for quick recognition of possible air contamination or vapor lock.
Perhaps the greatest criticism of carbon dioxide angiography is poor image quality. Sometimes one cannot perform the procedure solely with carbon dioxide and must rely on supplemental contrast media, albeit at reduced volumes in comparison to a traditional angiogram. The distal extremities also present a unique challenge because the gas must travel the farthest distance from the injection site. However, improvements in digital subtraction angiography software and techniques have greatly improved visualization in recent years. When the carbon dioxide is injected, there may be incomplete filling of the vessel, making it appear as though there are “holes” in the image. By digitally stacking multiple areas of the same vessel segment, a more complete picture can emerge. If this does not occur, one may mistake the “holes” for areas of stenosis.19
Pain at the injection site can result in inadvertent patient motion and further degrade image quality. An “explosive delivery” of gas may occur at the injection site, resulting in rapid expansion of the vessel and increased pain.10-21 Injections of local anesthesia at the injection site can help alleviate this. There is also a significant learning curve for the interventional cardiologist or vascular surgeon who wishes to perform a carbon dioxide angiogram. Currently, not all interventionalists are trained in this emerging technique. One doctor with over 20 years of experience commented on his own experiences, showing that the number of supplemental contrast media injections decreased from 2.5 to 0.6 over the span of 21 procedures.18
What The Literature Reveals
In 2014, Fujihara and colleagues conducted a prospective multicenter study of 98 patients with chronic kidney disease (with an average glomerular filtration rate of 35 mL/min) who presented with lesions of the renal, aortoiliac and superficial femoral arteries.20 The average volume of carbon dioxide was 281 ± 156 mL supplemented with only 15 ± 18 mL of iodinated contrast. Five percent of patients developed contrast-induced nephrotoxicity and 10 percent of patients reported transient leg pain. The authors reported a 97.9 percent success rate with ankle brachial indices (ABIs) improving from 0.66 to 0.83 three months after superficial femoral artery intervention.20
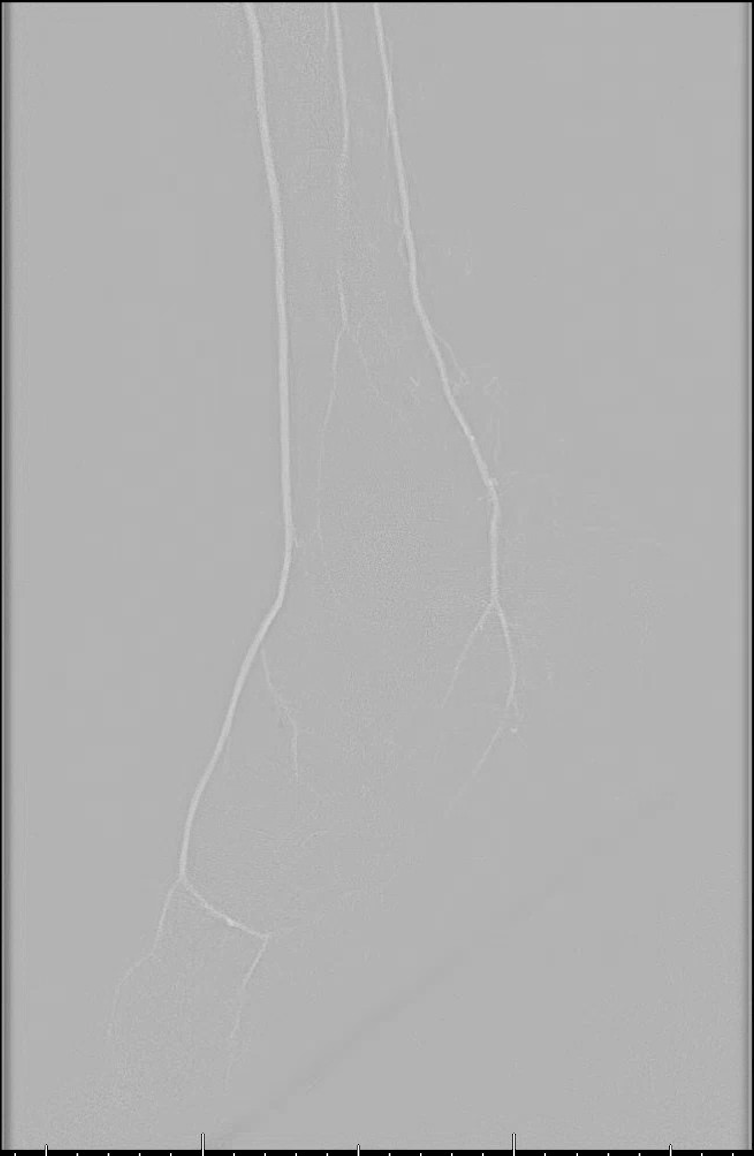 In 2016, Stegemann and coworkers performed a retrospective review of 154 patients who had traditional angiography and 37 patients who had carbon dioxide angiography with supplemental iodinated contrast.21 There were 29 cases of contrast-induced nephrotoxicity in the traditional angiography group in comparison with two cases in the carbon dioxide group. The amount of iodinated contrast used in the traditional angiography group was 113 ± 76 mL as opposed to only 34 ± 41 mL in the carbon dioxide angiography group. There was a 96 percent success rate in the traditional angiography group and a 100 percent success rate in the carbon dioxide group. The authors concluded that carbon dioxide angiography is a viable alternative but may be most useful in situations in which image accuracy is not critical, or when supplementation with iodinated contrast would not be absolutely contraindicated.
In 2016, Stegemann and coworkers performed a retrospective review of 154 patients who had traditional angiography and 37 patients who had carbon dioxide angiography with supplemental iodinated contrast.21 There were 29 cases of contrast-induced nephrotoxicity in the traditional angiography group in comparison with two cases in the carbon dioxide group. The amount of iodinated contrast used in the traditional angiography group was 113 ± 76 mL as opposed to only 34 ± 41 mL in the carbon dioxide angiography group. There was a 96 percent success rate in the traditional angiography group and a 100 percent success rate in the carbon dioxide group. The authors concluded that carbon dioxide angiography is a viable alternative but may be most useful in situations in which image accuracy is not critical, or when supplementation with iodinated contrast would not be absolutely contraindicated.
In 2016, Palena and colleagues conducted a prospective study of 36 patients with an average age of 74.8 years who had chronic kidney disease of stage 3 or greater (glomerular filtration rate ≤60 mL/min, the accepted definition across all studies) and critical limb ischemia.22 They initially performed a traditional angiogram with iodinated contrast medium so they could attain a comparison of image quality postoperatively but the intervention occurred solely with the guidance of carbon dioxide. The transcutaneous oxygen pressure improved from 11.8 ± 6.3 to 58.4 ± 7.6 mmHg. Carbon dioxide angiography had good sensitivity (92.3 percent) but slightly lower specificity (75 percent) and negative predictive value (63.1 percent), meaning that lesions could have been missed.22
Kusuyama and colleagues presented a case study of a 62-year-old male who had previously developed Stevens–Johnson syndrome in response to iodinated contrast.23 He required a stent of his superficial femoral artery for lower extremity pain and ulcers. A limitation of carbon dioxide angiograms is that one cannot digitally subtract stents out of the images. Therefore, it is difficult to assess if there are any residual areas of stenosis within the stent or if there has been dissection of the vessel wall from an oversized stent. The authors used intravascular ultrasound after deploying the stent to evaluate its positioning and efficacy. This technique uses an ultrasound probe on the end of the catheter wire as it is passed within the lumen of the vessel. They used 11 separate injections of 30 mL of carbon dioxide and successfully performed the intervention.
Similarly, Ephrem and coworkers reported a case study of a 92-year-old male with digital gangrene and a baseline creatinine of 2.7 mg/dL.24 The authors also performed a successful carbon dioxide angiogram and then assessed the stent with intravascular ultrasound.
In Conclusion
Carbon dioxide angiography is a viable alternative for patients who require vascular intervention but are unable to receive iodinated contrast either because of chronic kidney disease or allergy. As more interventionalists become trained in the technique and technology continues to improve, it may become more readily available across all centers. Foot and ankle specialists should be familiar with the indications, advantages and disadvantages of this technique, and become familiar with specialists in their area who perform it for appropriate referral when needed.
Dr. Crowell is a fourth-year resident at Temple University Hospital Podiatric Surgical Residency Program in Philadelphia.
Dr. Meyr is a Clinical Associate Professor within the Department of Surgery at Temple University School of Podiatric Medicine in Philadelphia.
References
- Pomposelli F. Arterial imaging in patients with lower extremity ischemia and diabetes mellitus. J Vasc Surg. 2010; 100(5):412-13.
- Malyar N, Furstenberg T, Wellmann J, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J. 2013; 34(34):2706-2714.
- Belch JJF, Topol EJ, Agnelli G, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003; 163(8):884-892.
- Duran C, Bismuth J. Advanced imaging in limb salvage. Methodist Debakey Cardiovasc J. 2012; 8(4):28-32.
- Graziani L, Silvestro A, Bertone V, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007; 33(4):453-460.
- Morcos SK, Thomsen HS. Adverse reactions to iodinated contrast media. Eur Radiol. 2001; 11(7):1267-1275.
- Agency for Healthcare Research and Quality. Contrast-induced nephropathy (CIN): current state of the evidence on contrast media and prevention of CIN. Available at: https://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2280&pageaction=displayproduct . Published Aug. 18, 2016.
- Hall KA, Wong RW, Hunter GC, et al. Contrast-induced nephrotoxicity: the effects of vasodilator therapy. J Surg Res. 1992; 53(4):317-20.
- Bashir R and Cooper CJ. Evaluation and medical treatment of peripheral arterial disease. Curr Opin Cariol. 2003; 18(6):436-443, 2003.
- Cho KJ. Carbon dioxide angiography: scientific principles and practice. Vasc Spec Int. 2015; 31(3):67-80.
- Hawkins IF. Carbon dioxide digital subtraction arteriography. AJR Am J Roentgenol. 1992; 139(1):19-24.
- Hawkins IF, Cho KJ, Caridi JG. Carbon dioxide in angiography to reduce the risk of contrast-induced nephropathy. Radiol Clin N Am. 2009; 47(5):813-825, 2009.
- Weaver FA, Pentecost MJ, Yellin AE. Carbon dioxide digital subtraction arteriography: a pilot study. Ann Vasc Surg. 1990; 4(5):437-41.
- Back MR, Caridi JG, Hawkins IF, Seeger JM. Angiography with carbon dioxide (CO2). Surg Clin N Am. 1998; 78(4):575-591.
- Caridi JG, Hawkins IF. CO2 digital subtraction angiography: potential complications and their prevention. J Vasc Interv Radiol. 1997; 8(3):383-391.
- Rundback JH, Shah PM, Wong J, Babu SC, Rozenblit G, Poplausky MR. Livedo reticularis, rhabdomyolysis, massive intestinal infarction, and death after carbon dioxide arteriography. J Vasc Surg. 1997; 26(2):337-340.
- Dogan M, Un H, Aparci M, Kardesoglu E. Carbon dioxide angiography: to be or not to be an alternative? Angiology. 2016; 67(10):973.
- Giordano A, Messina S, Polimeno M, et al. Peripheral diagnostic and interventional procedures using an automated injection system for carbon dioxide (CO2): case series and learning curve. Heart Lung Vessels. 2015; 7(1):18-26.
- Lang EV, Gossler AA, Fick LJ, et al. Carbon dioxide angiography: effect of injection parameters on bolus configuration. J Vasc Interv Radiol. 1999; 10(1):41-9.
- Fujihara M, Kawasaki D, Shintani Y, et al. Endovascular therapy by CO2 angiography to prevent contrast-induced nephropathy in patients with chronic kidney disease: a prospective multicenter trial of CO2 angiography registry. Catheter Cardiovasc Interv. 2015; 85(5):870-877.
- Stegemann E, Tegtmeier C, Bimpong-Buta NY, et al. Carbon dioxide-aided angiography decreases contrast volume and preserves kidney function in peripheral vascular interventions. Angiology. 2016; 67(9):875-881.
- Palena LM, Diaz-Sandoval LJ, Candeo A, et al. Automated carbon dioxide angiography for the evaluation and endovascular treatment of diabetic patients with critical limb ischemia. J Endovasc Ther. 2015; 23(1):40-48.
- Kusuyama T, Iida H, Mitsui H. Intravascular ultrasound complements the diagnostic capability of carbon dioxide digital subtraction angiography for patients with allergies to iodinated contrast medium. Catheter Cardiovasc Interv. 2012; 80(6):E82-E86.
- Ephrem G, Garikipati S, Hanson ID. The fluoro-less and contrast-less peripheral endovascular intervention: halfway there. Cardiovasc Revasc Med. 2016; 17(6):418-420.











