Assessing Collagen-Based Modalities For Diabetic Foot Ulcerations
Collagen-based products reportedly promote increased fibroblast proliferation and inhibit excessive matrix metalloproteinases in addition to other benefits for diabetic foot ulcerations. With this in mind, these authors take a closer look at the research on collagen modalities, comparing various established and emerging options.
 An estimated 415 million individuals have diabetes worldwide, a number that is expected to increase to 642 million by 2040.1-3 People affected by diabetes have a 25 percent lifetime risk of developing a diabetic foot ulceration (DFU). Annually, approximately $480 billion goes toward the treatment of DFUs.1-3
An estimated 415 million individuals have diabetes worldwide, a number that is expected to increase to 642 million by 2040.1-3 People affected by diabetes have a 25 percent lifetime risk of developing a diabetic foot ulceration (DFU). Annually, approximately $480 billion goes toward the treatment of DFUs.1-3
Diabetes notoriously impairs the immune system and impedes the healing process. Patients with diabetes are more susceptible to developing non-healing foot infections, many of which lead to complex medical comorbidities and costly detriments. Consequently, diabetic foot infections are the most common cause of non-traumatic foot amputations in the United States.4,5 Fifteen percent of DFUs result in more proximal amputations, corresponding to high rates of mortality within five to eight years of the initial amputation.4,5
Diabetic foot ulcers commonly result from a combination of neuropathy, vasculopathy, pressure and/or infection. Due to hyperglycemic states that occur in individuals with diabetes, glycoproteins develop and basement membranes thicken, leading to endothelial proliferation, decreased vessel permeability, and altered cell migration.6 Extracellular matrix degradation and faulty cellular regulation induce high concentrations of inflammatory cytokines within the wound environment.1,3 These processes cause cellular senescence and induce increased protease enzymes. Additionally, this cascade degrades growth factors, receptors and essential matrix and support structures. Angiogenesis decreases and an environment with an imbalance of matrix metalloproteinases (MMPs) to tissue inhibitor metalloproteinases (TIMPs) develops.7-10
Diabetic foot ulcers are challenging to treat due to impairments in healing from prolonged inflammatory responses, defective extracellular matrices, higher levels of protease enzymes and compromised blood vessels. A longer chronicity of DFUs leads to a higher probability that the patient with a DFU will develop complications and infections both locally and systemically, leading to potential loss of limb or life.11
 What You Should Know About The Efficacy Of Collagen
What You Should Know About The Efficacy Of Collagen
For these reasons, it is critically important to treat DFUs appropriately with modalities proven to be effective. There are a wide variety of DFU treatment options including sharp tissue debridement, inflammation and infection control, local wound care, offloading, larval therapy, hyperbaric oxygen therapy, negative pressure wound therapy, and advanced skin substitutes and grafts.2,10,12,13 Collagen-based modalities have emerged as widely popular and effective treatments for DFUs.
Collagen is a triple stranded helical structure and major protein comprising the extracellular matrix. Composed primarily of keratinocytes and fibroblasts, collagen-based modalities work by providing a biological structural scaffold matrix to support extracellular components.2,10,13 Collagen-based dressings function by providing a viable extracellular matrix, which is compromised in DFUs. This scaffold ultimately promotes increases of fibroblast proliferation and permeation in addition to helping mediate cell migration and organization. It preserves leukocytes and macrophages, and inhibits excessive MMPs present in chronic DFUs, ultimately assisting in the healing process.14,15
Key Insights On Two Bioengineered Products
When it comes to treating DFUs, there are a variety of collagen-based modalities that derive from several sources and are accessible in different formulations, sizes and compositions. Apligraf (Organogenesis) is a bioengineered living bilayer product composed of an epidermal layer of keratinocytes and a dermal layer of fibroblasts deriving from neonatal foreskin and placed in a bovine type I collagen matrix.3 The dermoinductive product works by providing the growth factors and matrix that are defective in DFUs.
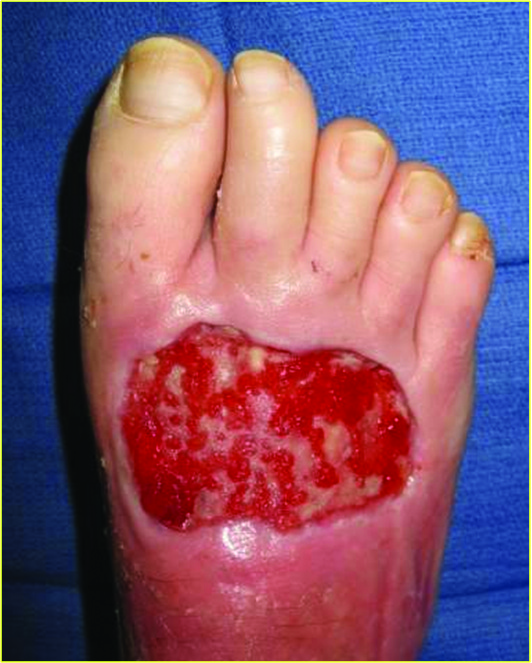
 Kirsner and colleagues evaluated Apligraf application on 163 DFUs from 155 patients with an average wound area of 6.0 ± 5.5 cm2 and wound duration of 4.4 ± 2.6 months.9 Sixty-five percent of patients required multiple applications but overall, there was a 70 percent improvement in wound closure in 12 weeks. The study authors concluded that DFUs treated with Apligraf had higher, faster healing rates. They also found an increase in the probability of healing by 97 percent in comparison to dehydrated amniotic membranes.
Kirsner and colleagues evaluated Apligraf application on 163 DFUs from 155 patients with an average wound area of 6.0 ± 5.5 cm2 and wound duration of 4.4 ± 2.6 months.9 Sixty-five percent of patients required multiple applications but overall, there was a 70 percent improvement in wound closure in 12 weeks. The study authors concluded that DFUs treated with Apligraf had higher, faster healing rates. They also found an increase in the probability of healing by 97 percent in comparison to dehydrated amniotic membranes.
Apligraf is approved by the Food and Drug Administration (FDA) with an indication for use “with standard diabetic foot ulcer care for the treatment of full-thickness neuropathic diabetic foot ulcers of greater than three weeks’ duration, which have not adequately responded to conventional ulcer therapy and which extend through the dermis but without tendon, muscle, capsule or bone exposure.”16
Dermagraft (Organogenesis) is another bioengineered dermal product for the treatment of DFUs. It is a cryopreserved human fibroblast-derived dermal substitute. Dermagraft is composed of fibroblasts, extracellular collagen matrix and a bioabsorbable polyglactin mesh scaffold. The combination of metabolically active cells and fibroblasts incorporated into the mesh help stimulate epithelialization, allowing it to be another dermoinductive product.3 Dermagraft is FDA approved for full thickness DFUs present for over six weeks, which extend through the dermis but do not involve tendon, muscle, joint capsule or bone.11,12,17,18
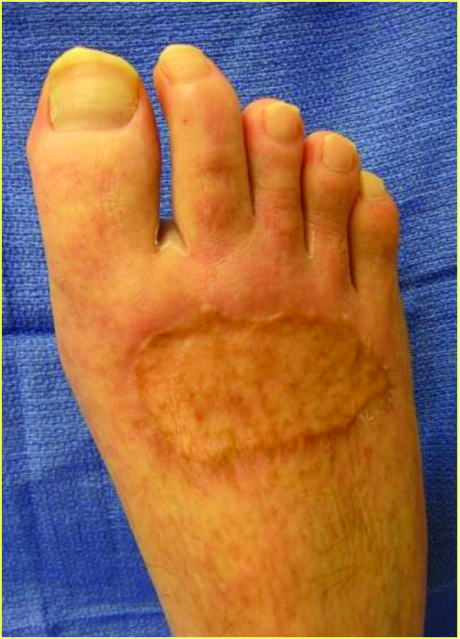
Marston and coworkers studied the effects of Dermagraft versus conventional therapy (wet to dry dressing) alone in 245 patients with chronic DFUs in a randomized controlled study.19 The results demonstrated that treatment with Dermagraft produced a significantly greater proportion (30 percent) of fully healed ulcers in comparison to the control group (18 percent). Additionally, the Dermagraft group had 91 percent closure by week 12 in comparison to 78 percent for the control group. The photos above demonstrate a wound treated with Dermagraft.
Assessing The Impact Of A Wound Dressing As A Protease Inhibitor In Treating DFUs
Promogran (Systagenix) is a collagen-based DFU treatment that chemically modifies the wound environment by acting as a protease inhibitor.7,20 Promogran is a hexagonal graft that is 55% collagen and 45% oxidized regenerated cellulose, both of which work to bind and inactivate MMPs and elastase within the wound bed to assist with the release of positive growth factors. Promogran Prisma (Systagenix) is a version of Promogran that helps reduce bacterial growth with the addition of 1% silver.21,22
Lobmann and colleagues studied 33 patients with DFUs treated with Promogran versus a control dressing.23 After an eight-day period of DFU treatment, the authors performed three separate tissue biopsies to analyze protease levels. The study showed the Promogran treatment had greater reduction in wound diameter in comparison to the control group (16 percent) and a significant decrease in the MMP-9/TIMP-2 ratio, likely due to MMPs binding to collagen matrix.
A Closer Look At Dermoconductive Solutions
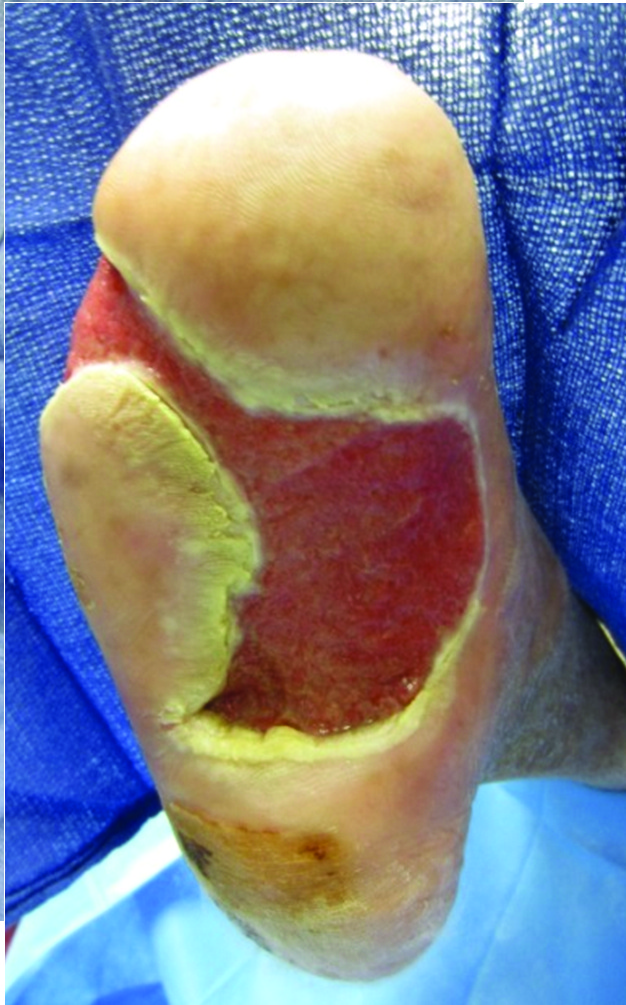
Another collagen-based modality for DFU is Integra Bilayer Wound Matrix (Integra LifeSciences), which one can use for both full-thickness and deep partial thickness DFUs. The Integra graft is similar to Apligraf in that it is a bilayer membrane but it functions as an acellular dermoconductive product.3 The epidermal replacement layer is composed of a semi-permeable thin silicone layer while the dermal component is composed of cross-linked bovine type I collagen with glycosaminoglycan and shark chondroitin-6-sulfate. This configuration allows the epidermal layer to control moisture, graft flexibility and infection resistance.
The dermal layer provides a scaffold for cellular invasion and growth. The Integra Bilayer Wound Matrix graft is available in both non-meshed and meshed configurations that assist in the reduction of hematoma and seroma formation.16,24-26 The photos below demonstrate an ulceration treated with this graft.
As of January 2016, Omnigraft Dermal Regeneration Matrix (Integra LifeSciences) is FDA approved to treat “certain diabetic foot ulcers that last for longer than six weeks and do not involve exposure of the joint capsule, tendon or bone, when used in conjunction with standard diabetic ulcer care.”27
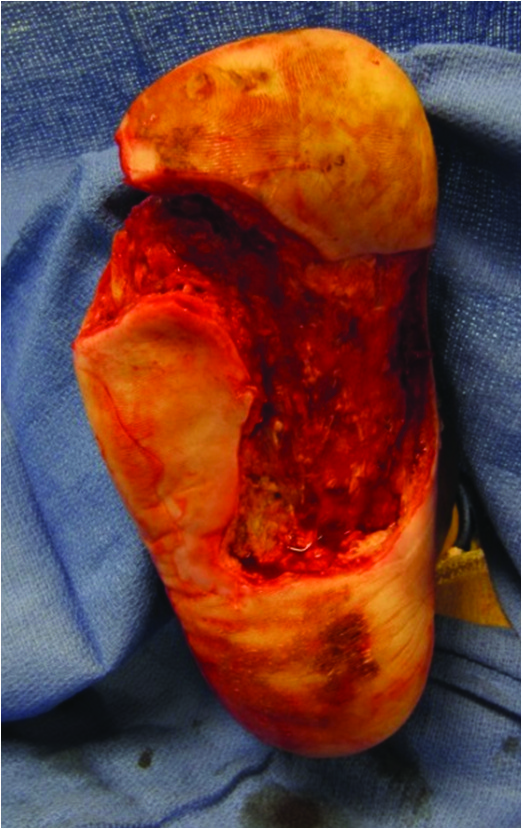 In 2015, Driver and coworkers published a detailed clinical trial of the Integra Dermal Regeneration Template (now marketed as Omnigraft) for DFUs.6 The study had two distinct phases and enrolled 307 patients, each with at least one DFU. The first phase consisted of a 14-day segment with patients in the study receiving 0.9% sodium chloride gel with a secondary dressing and offloading of the ulceration. Upon completion of the first phase, patients with less than 30 percent re-epithelialization progressed to phase two with randomization to a control group with 0.9% sodium chloride gel or treatment group with Integra bilayer graft.
In 2015, Driver and coworkers published a detailed clinical trial of the Integra Dermal Regeneration Template (now marketed as Omnigraft) for DFUs.6 The study had two distinct phases and enrolled 307 patients, each with at least one DFU. The first phase consisted of a 14-day segment with patients in the study receiving 0.9% sodium chloride gel with a secondary dressing and offloading of the ulceration. Upon completion of the first phase, patients with less than 30 percent re-epithelialization progressed to phase two with randomization to a control group with 0.9% sodium chloride gel or treatment group with Integra bilayer graft.
During the 16-week follow-up, patients who received the Integra graft had a significantly greater complete closure rate (51 percent) versus the control group (32 percent).6 The mean time to closure was 43 days versus 78 days for the control group and weekly wound reduction size was 7.2 percent for the Integra graft group versus 4.8 percent for the control group.
Another collagen-based modality with dermoconductive properties is Graftjacket Regenerative Tissue Matrix (Wright Medical), a collagen-based cadaveric fenestrated allograft.3 The acellular dermal scaffold is primarily composed of collagen, elastin, hyaluronan, fibronectin and blood vessel channels.25,26 The cryogenically preserved matrix has a two-year shelf life and is available in a thickness of 0.38 to 1.02 mm. Graftjacket Xpress (Wright Medical), a collagen modality with a similar composition and mechanism of action as Graftjacket Regenerative Tissue Matrix, offers a 2 cc injectable soft tissue scaffold, which is ideal for irregularly shaped wounds and wounds with tunneling and undermining.26
In 2015, Reyzelman published a comprehensive literature review and analysis of the effectiveness of Graftjacket Regenerative Tissue Matrix in DFUs.28 The comparative analysis of three fundamental controlled clinical trials involving 154 patients with DFUs revealed that ulcers treated with Graftjacket had a nearly fourfold increase in the probability of wound healing in comparison to moist wound care. Additionally, there was a statistically significant decrease in mean wound healing time of 1.7 weeks with Graftjacket in comparison to traditional moist-to-dry dressing changes.
Why Adding Alginate Increases Healing Potential
Fibracol Plus (Systagenix) is a collagen-based DFU modality with a 90% collagen and 10% alginate composition. The addition of alginate helps absorb excessive wound moisture and exudates. Fibracol is non-adherent, facilitating autolytic debridement to achieve formation of granulation tissue.
Donaghue and colleagues performed a randomized control study looking at a collagen-alginate dressing, Fibracol, versus saline-moistened gauze in 75 patients with DFUs.29 The authors noted a mean percent reduction in wound area of 80.6 percent in the group treated with Fibracol and 61.1 percent in the control group. Additionally, 78 percent of patients in the collagen-alginate group experienced a more than 75 percent reduction in wound size with 48 percent achieving complete healing in comparison to 36 percent in the control group.
Further Insights On Innovative Collagen Products
TheraSkin (Soluble Systems) is a split-thickness allograft harvested within 24 hours post-mortem and cryopreserved to maintain living cellular characteristics. As harvested human skin, the graft has large amounts of collagen, cytokines and growth factors typically found on live skin. A study by DiDomenico and colleagues comparing 12 wounds treated with TheraSkin to 17 wounds treated by Apligraf found a higher closure rate with the TheraSkin treatment group.30
 Biovance (Alliqua BioMedical) is a collagen-based wound covering produced from decellularized, dehydrated human amniotic membrane (DDHAM). A randomized control trial by Smiell and coworkers of 179 chronic wounds demonstrated “the safety and clinical benefit of DDHAM to support wound closure across a variety of chronic wound types and patient conditions in real-world environments.”31
Biovance (Alliqua BioMedical) is a collagen-based wound covering produced from decellularized, dehydrated human amniotic membrane (DDHAM). A randomized control trial by Smiell and coworkers of 179 chronic wounds demonstrated “the safety and clinical benefit of DDHAM to support wound closure across a variety of chronic wound types and patient conditions in real-world environments.”31
PuraPly (Organogenesis) is a purified collagen matrix with a polyhexamethylene biguanide hydrochloride (PHMB) antimicrobial agent. The product is indicated for a variety of wound types, including diabetic foot ulcers. The polyhexamethylene biguanide hydrochloride adds broad antimicrobial coverage and reduction of bacterial loads within the wound. It combines broad antimicrobial antiseptic spectrums with low toxicity and high tissue compatibility.32
Several other collagen products are available for use on wounds. These products include Oasis (Smith and Nephew), EpiFix (MiMedx), Epicel (Vericel), OrCel (Forticell Bioscience), Biobrane (Smith and Nephew), and EZ Derm (Mölnlycke Health Care).3,9,22 Collagen products offer wound healing benefits with limited drawbacks. Products may have contraindications depending on formulation, including known sensitivities to bovine, porcine or avian-based products.
The major limitation with collagen products is overall research. While there is current literature about the success of collagen-based treatments for DFU, there is very limited research comparing the different types of collagen treatments and their effectiveness with different wound varieties. Future research should help expand our knowledge of these collagen-based treatments and continue to show their numerous advantages for DFUs with a variety of sizes, depths and wound characteristics.
In Conclusion
There are a variety of collagen-based modality treatments for diabetic foot ulcerations. Each modality is based on different general compositions and offers features to enhance wound healing, demonstrated by significant advantages in current evidence-based literature. In comparison to the current standard of DFU care without the use of collagen-based products, these modalities offer improved treatment options for DFUs, decreasing the time for wound closure, increasing healing capabilities and improving overall limb salvage and mortality rates. Although collagen-based treatments are effective, suboptimal wounds have decreased chances for success.
The collagen-based products effectively replace and supplement the extracellular matrix, provide essential growth factors, and promote cellular proliferation and angiogenesis. One should use collagen as an adjunct therapy to traditional wound care, including sharp debridement, offloading, infection control and adequate perfusion, to assist in achieving maximum benefits.
Dr. Gazes is a second-year resident in the Department of Podiatric Surgery at Yale New Haven Hospital.
Dr. Morra is a first-year resident in the Department of Podiatric Surgery at Yale New Haven Hospital.
Dr. Blume is an Assistant Clinical Professor of Surgery in the Department of Surgery and an Assistant Clinical Professor of Orthopaedics and Rehabilitation in the Department of Orthopaedics, Section of Podiatric Surgery at the Yale University School of Medicine in New Haven, Ct. He is a Fellow of the American College of Foot and Ankle Surgeons.
References
- International Diabetes Foundation Diabetes Atlas, Sixth Edition. Available at https://www.idf.orghttps://s3.amazonaws.com/HMP/hmp_ln/imported/EN_6E_Atlas_Full_0.pdf .
- Snyder RJ, Kirsner RS, Warriner RA 3rd, Lavery LA, Hanft JR, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage. 2010; 56(4 Suppl.):S1–24.
- Garwood C, Steinberg J, Kim P. Bioengineered alternative tissues in diabetic wound healing. Clin Podiatr Med Surg. 2015; 32(1):121-133.
- Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin Infect Dis. 2012; 54(12):132–173.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004; 39(7):885–910.
- Driver V, Lavery L, Reyzelman A, Dutra T. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Rep Regen. 2015; 23(6):891-900.
- Brett D. A review of collagen and collagen- based wound dressings. Wounds. 2008; 20(12):347-56.
- Gould LJ. Topical collagen-based biomaterials for chronic wounds: rationale and clinical application. Adv Wound Care. 2016; 5(1):19-31.
- Kirsner RS, Sabolinski ML, Parsons NB, Skornicki M, Marston WA. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen. 2015; 23(5):737–744.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment: a meta-analysis. Diabetes Care. 1999; 22(5):692–5.
- Braun L, Fisk W, Lev-Tov H, Kirsner R, Isseroff R. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol. 2014; 15(3):267-81.
- Wu L, Norman G, Dumville JC, O’Meara S, Bell-Syer SEM. Dressings for treating foot ulcers in people with diabetes: an overview of systematic reviews. Cochrane Database Syst Rev. 2015 July 14; 7:CD010741.
- Bakker K, Apelqvist J, Lipsky B, Van Netten J. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016; 32(Suppl 1):2-6.
- Rizzi SC, Upton Z, Bott K, Dargaville TR. Recent advances in dermal wound healing: biomedical device approaches. Expert Rev Med Devices. 2010; 7(1):143-54.
- Karri V, Kuppusamy G, Takkuri S, Yamjala K, et al. Current and emerging therapies in the management of diabetic foot ulcers. Curr Med Res Opin. 2016; 32(3):519-43.
- 2015 Biologic Device Application Approvals. Available at: https://www.fda.gov/biologicsbloodvaccines/developmentapprovalprocess/biologicalapprovalsbyyear/ucm434965.htm . Accessed February 14, 2016.
- Reyzelman A. Human acellular dermal wound matrix for treatment of dfu: literature review and analysis. J Wound Care. 2015; 24(3):128-34.
- Dermagraft® - P000036. Available at: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm085085.htm . Accessed February 14, 2016.
- Marston W, Hanft J, Norwood P, Pollak R. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers. Results of a prospective randomized trial. Diabetes Care. 2013; 26(6):1701-1705.
- Ulrich D, Smeets R, Unglaub F, Woltje M, Pallua N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J Wound Ostomy Continence Nurs. 2011; 38(5):522–8.
- Cullen B, Ivins N. Promogran & Promogran Prisma made easy. Wounds Int. 2010; 1(3):1-6.
- Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):1-28.
- Lobmann R, Zemlin C, Motzkau M, et al. Expression of metalloproteinases and growth factors in diabetic wounds treated with a protease absorbent dressing. J Diabetes Complications. 2006; 20(5):329-35.
- Holmes C, Wrobel J, MacEachern M, Boles B. Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab Syndr Obes. 2013; 6:17-29.
- Helary C, Abed A, Mosser G, Louedec L, et al. Evaluation of dense collagen matrices as medicated wound dressing for the treatment of cutaneous chronic wounds. Biomater Sci. 2015 3(2):373-382.
- Cereceres S, Touchet T, Browning M, Smith C, Rivera J, et al. Advances Wound Care. 2015; 4(8):444-456.
- FDA approves Integra Omnigraft Dermal Regeneration Matrix to treat diabetic foot ulcers. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm480564.htm. Retrieved: February 14th, 2016.
- Reyzelman AM, Barazov I. Human acellular dermal wound matrix for treatment of DFU: literature review and analysis. J Wound Care. 2015; 24(3):128-34.
- Donaghue VM, Chrzan JS, Rosenblum BI, Giurini JM, Habershaw GM, Veves A. Evaluation of a collagen-alginate wound dressing in the management of diabetic foot ulcers. Advances Wound Care. 1998;11(3):114–9.
- DiDomenico L, Emch K, Landsman AR, Landsman A. A prospective comparison of diabetic foot ulcers treated with either cryopreserved skin allograft or bioengineered skin substitute. Wounds. 2011;23(7):184-189.
- Smiell J, Treadwell T, Hahn H, Hermans M. Real-world experience with a decellularized dehydrated human amniotic membrane allograft. Wounds. 2015;27(6):158-169.
- Hübner N, Kramer A. Review of the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol Physiol. 2010;23(Suppl):17-27.











