Amniotic Membrane: Can It Facilitate Healing?
 The avascular, low immunogenic, anti-inflammatory, anti-scarring and wound healing properties of amniotic membrane make it valuable in a wide range of regenerative medicine applications. These authors review the clinical and scientific evidence pertaining to the contemporary use of amniotic membrane for the treatment of chronic lower extremity wounds.
The avascular, low immunogenic, anti-inflammatory, anti-scarring and wound healing properties of amniotic membrane make it valuable in a wide range of regenerative medicine applications. These authors review the clinical and scientific evidence pertaining to the contemporary use of amniotic membrane for the treatment of chronic lower extremity wounds.
Throughout the world, non-healing wounds impart significant challenges on both the people affected and their healthcare providers. When wounds fail to heal with standard therapies over a reasonable period of time, clinicians may select advanced modalities, such as amniotic membrane, with a clear scientific rationale to promote accelerated healing. However, advanced treatments may incur sizable financial ramifications so when evaluating these wound care products it is important to examine factors in addition to overall effectiveness. These factors include time to healing, the amount of product used and wasted, as well as ease of product storage and application techniques, which can influence administrative and clinical costs.
Reports on the clinical use of amniotic membrane span over a century but widespread use of the material was fraught with issues related to obtaining and handling the membrane and concerns relative to disease transmission.1 Accordingly, fresh amniotic membrane is not practical for routine clinical use. However, recent advances in tissue processing and preservation techniques have yielded commercially available amniotic membrane allografts. With this in mind, let us take a closer look at the clinical and scientific evidence regarding the ability of amniotic membrane-derived products to facilitate wound healing.
Amniotic membrane lines the amniotic cavity and protects the fetus during pregnancy, providing physical support and serving as a metabolic filter. The amniotic membrane regulates the transport of water and soluble materials in addition to the production of growth factors, cytokines and other bioactive molecules.2 The amniotic membrane is metabolically active and continually remodels and grows to accommodate the developing fetus. This process is controlled by growth factors, cytokines, chemokines and related regulatory factors produced by the endogenous cells in the amniotic membrane.
Amniotic membrane consists of two distinct layers: the amnion, which is the inner layer closest to the fetus; and the chorion, which is the outer layer closest to the mother’s uterus. The amnion consists of a layer of epithelial cells anchored to a basement membrane by a compact collagen-rich tissue. The chorion primarily consists of dense collagen fibers in an interfibrillar matrix containing proteoglycans and elastic fibers. The chorion layer is four to five times thicker than the amnion layer.3 Neither the amnion nor the chorion is vascular.
Amniotic membrane is immunologically privileged and exhibits anti-inflammatory properties as well as being a reservoir of multiple growth factors involved with tissue growth and regeneration. Such properties confer remarkable therapeutic potential on amniotic membrane for wound healing, tissue repair and regenerative therapy.1,4-7 Growth factors contained within amniotic membrane include epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), keratinocyte growth factor (KGF), vascular endothelial growth factor (VEGF), transforming growth factors (TGFs), nerve growth factor (NGF) and many chemokines known to be important for the healing of both acute and chronic wounds.8,9
 Although both the amnion and chorion layers of the amniotic membrane contain growth factors, chorion has four to five times greater growth factor content than amnion per equivalent surface area.3 In an in vitro study comparing growth factor levels between single-layered (amnion) and multilayered (amnion/chorion) allograft, the total cytokine content contributed by chorion was higher than that contributed by amnion alone.3 In all cases, bilayered (amnion plus chorion) allograft contained significantly more growth factors than the single layer amnion grafts.
Although both the amnion and chorion layers of the amniotic membrane contain growth factors, chorion has four to five times greater growth factor content than amnion per equivalent surface area.3 In an in vitro study comparing growth factor levels between single-layered (amnion) and multilayered (amnion/chorion) allograft, the total cytokine content contributed by chorion was higher than that contributed by amnion alone.3 In all cases, bilayered (amnion plus chorion) allograft contained significantly more growth factors than the single layer amnion grafts.
Several amniotic membrane products are available for the clinician to use in wound management. These products may be dehydrated or cryopreserved, and may contain amnion alone or amnion and chorion. It is important that clinicians are aware of the composition of the allograft they are using and the clinical evidence, if any, related to each product. The majority of scientific and clinical evidence in the peer-reviewed literature related to currently available amniotic membrane based products for use in wound healing relates to one particular dehydrated human amnion/chorion (dHACM) allograft (EpiFix, MiMedx Group) while there is one paper on the use of a cryopreserved single layer amnion product (Grafix, Osiris Therapeutics).3,10-26
Current Insights On The Potential Of Dehydrated Human Amnion/Chorion Membrane Allograft
Dehydrated human amnion/chorion membrane allografts consist of both amnion and chorion layers of the amniotic membrane deriving from the placenta.27-29 Isolating the amniotic membrane from placenta and preserving it with a proprietary Purion Process, which involves gentle cleansing of the layers and dehydration of the tissue, results in an allograft with a five-year shelf-life under ambient conditions. The source of amniotic membrane for dHACM is donor placenta obtained following cesarean delivery with informed consent from women with acceptable infectious disease test results as regulated by the Food and Drug Administration’s (FDA) Good Tissue Practice requirements and the American Association of Tissue Banks. The Purion Process retains the native composition of extracellular matrix and signaling molecules, and preserves bioactivity.
 Researchers have shown that the growth factors present in dHACM induce human dermal fibroblast proliferation, which is relevant to wound healing.10 The array of cytokines preserved in dHACM is in part responsible for its therapeutic efficacy in treating chronic wounds by orchestrating a “symphony of signals” to promote healing.10
Researchers have shown that the growth factors present in dHACM induce human dermal fibroblast proliferation, which is relevant to wound healing.10 The array of cytokines preserved in dHACM is in part responsible for its therapeutic efficacy in treating chronic wounds by orchestrating a “symphony of signals” to promote healing.10
Studies of amniotic membrane using dHACM reveal the ability of the tissue to recruit multiple stem cells relevant to wound repair and regeneration. Both stromal cell derived factor 1 (SDF-1) and CXC chemokine receptor type 4 (CXCR4), which facilitate stem cell recruitment and homing factors, are present in dHACM. In vitro studies confirmed the dehydrated allograft can act as a “stem cell magnet” to stimulate the migration of mesenchymal stem cells.10 In vivo studies have also shown stem cell homing to sites of neovascularization, reflecting their role as endothelial progenitor cells.10-12 The dHACM actually releases factors that recruit endogenous stem cells, suggesting true regenerative capability when clinicians use dHACM in wound management.
Research has also demonstrated the ability of dHACM to induce angiogenesis.12 Multiple pro-angiogenic factors are present in and released by dHACM.12 Studies using the conditioned media of dHACM found that allograft stimulated the upregulated production of endogenous angiogenic factors by endothelial cells, supporting a magnified paracrine effect to stimulate wound angiogenesis.12
These defined mechanisms provide a scientific basis to explain the clinical benefits of using dHACM to heal wounds.
Examining The Clinical Evidence For The Use Of Amniotic Membrane To Facilitate Healing
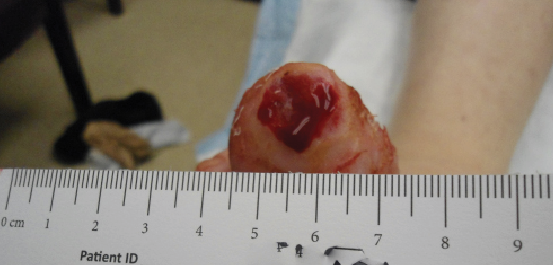
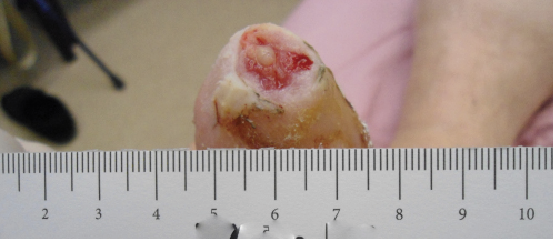
A review of contemporary clinical evidence regarding the use of dHACM to facilitate healing reveals a myriad of information in the peer-reviewed literature ranging from case reports, case series and retrospective studies to randomized controlled trials and studies of comparative effectiveness.13-25 Published case reports on the use of dHACM as a wound treatment include successful healing of a plantar full-thickness pedal burn and an unusual case of a premature infant with congenital candidiasis who developed severe skin and tissue loss. In both cases, there was healing within four weeks of one dHACM allograft application.13-14 In a case series of three recalcitrant diabetic foot ulcers that had not decreased in size by 50 percent after four weeks of standard care, all went on to completely heal after dHACM therapy began.15
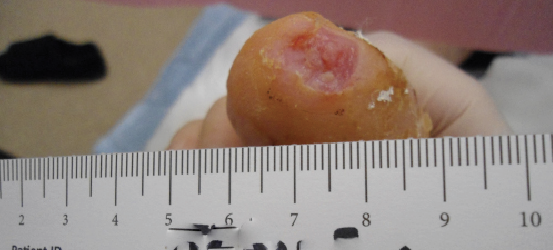 Another case series described four patients with refractory wounds who had initial referrals for a definitive plastic surgery procedure (i.e. flap reconstruction surgery) but received dHACM instead.16 Healing was evident in a variety of wound types with one to three dHACM applications, eliminating the need for surgical intervention. In each case, a dramatic reduction in wound size occurred after the first application of dHACM. In yet another retrospective case series of five patients with recalcitrant wounds (who failed to heal at least 50 percent of their wound after one month of standard wound care), ulcers subsequently treated with dHACM demonstrated improved healing and a change in healing trajectory in comparison to those documented prior to application.17
Another case series described four patients with refractory wounds who had initial referrals for a definitive plastic surgery procedure (i.e. flap reconstruction surgery) but received dHACM instead.16 Healing was evident in a variety of wound types with one to three dHACM applications, eliminating the need for surgical intervention. In each case, a dramatic reduction in wound size occurred after the first application of dHACM. In yet another retrospective case series of five patients with recalcitrant wounds (who failed to heal at least 50 percent of their wound after one month of standard wound care), ulcers subsequently treated with dHACM demonstrated improved healing and a change in healing trajectory in comparison to those documented prior to application.17
In early 2013, Zelen and colleagues published results from the first prospective, randomized, single-center clinical trial comparing healing characteristics of diabetic foot ulcers treated with dHACM versus standard of care.19 Patients received either standard of care alone or standard of care with the addition of dHACM. Researchers evaluated wound size reduction and rates of complete healing after four and six weeks. Significant differences were evident in wound reduction at four weeks with wounds reducing in size by a mean of 32 percent ± 43.7 percent in 12 patients in the standard of care arm versus 97.1 percent ± 7 percent for 13 patients receiving dHACM. At six weeks, the difference between the groups was even greater. In the standard of care group, the mean wound size had increased 1.8 percent ± 0.3 percent versus a mean wound size reduction of 98.4 percent ± 5.8 percent in the dHACM group.
After four and six weeks of treatment, the overall healing rates with dHACM were 77 percent and 92 percent respectively in comparison to 0 percent and 8 percent respectively with standard of care.19 In the study protocol, initial sample size calculations were based on historic healing rates with other advanced wound care products but due to the large, statistically significant differences between the study groups, physicians decided to terminate the study early for ethical reasons despite the small study size.
A second investigation focused on 11 patients whose wounds remained unhealed after six weeks of receiving standard of care and wound offloading in the initial study.20 In the crossover study design, these patients with non-healing wounds had treatment with dHACM. Confirming results of the initial study, a similar accelerated healing effect occurred in these individuals. At the initiation of dHACM treatment, wounds were present for a mean of 21.1 ± 12.4 weeks and the mean wound size was 4.7 ± 5 cm2. Complete healing occurred in 55 percent of the wounds by four weeks, 64 percent by six weeks and 91 percent by 12 weeks with biweekly dHACM applications.
Even following the use of advanced wound care products, wounds may reoccur. An additional follow-up evaluation involved all patients who received dHACM and healed in initial and crossover studies.21 The evaluation of 18 wounds healed with dHACM occurred nine to 12 months after primary healing. Seventeen of 18 ulcers remained fully healed, suggesting the durability of wound repair with dHACM.
How Often Should One Apply dHACM?
In these initial studies, patients had dHACM application every two weeks. A subsequent study examined if weekly application of dHACM further reduced healing time.22 The prospective, randomized, comparative study involved 40 patients with diabetic foot ulcers of at least four weeks duration that did not heal with standard treatment. Patients in the study received either weekly or biweekly application of dHACM in addition to non-adherent, moist wound healing, compression wraps and offloading. Overall, during the 12-week study period, 37 out of the 40 dHACM-treated ulcers completely healed. The number of grafts required for healing was similar in both groups but the mean time to heal was only 2.4 ± 1.8 weeks in the weekly group in comparison to 4.1 ± 2.9 weeks in the biweekly group. By four weeks, complete healing occurred in 90 percent of the wounds in the weekly application group versus 50 percent of wounds in the biweekly application group.
In summary, the difference in healing rates for weekly versus biweekly applications of dHACM demonstrated a clear clinical preference for weekly application of the material as those wounds receiving weekly application of dHACM healed over 40 percent faster. The faster healing with weekly application also shows an economic advantage given the patients receiving weekly application required fewer treatment visits and dressing changes at the wound healing center.
Are All Amniotic Membrane Products Equally Effective?
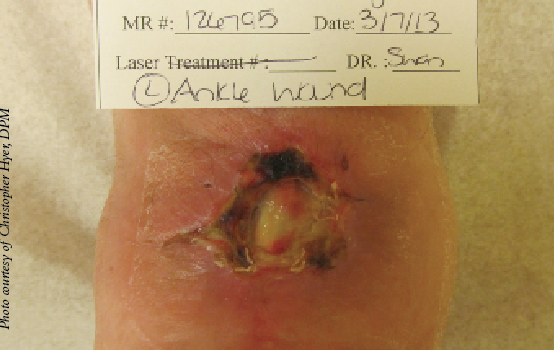 A randomized, prospective study of single-layer, amnion only (Grafix), cryopreserved placental membrane applied weekly to chronic diabetic foot ulcers versus standard care with debridement, offloading and non-adherent dressings also supports the use of amniotic membrane for wound healing.26 Rates of complete healing (62 percent versus 21.3 percent) and median time to healing (42 days versus 69.5 days) were significantly better in patients treated with cryopreserved placental membrane in comparison with controls.
A randomized, prospective study of single-layer, amnion only (Grafix), cryopreserved placental membrane applied weekly to chronic diabetic foot ulcers versus standard care with debridement, offloading and non-adherent dressings also supports the use of amniotic membrane for wound healing.26 Rates of complete healing (62 percent versus 21.3 percent) and median time to healing (42 days versus 69.5 days) were significantly better in patients treated with cryopreserved placental membrane in comparison with controls.
To date, there are no clinical studies comparing treatment outcomes of dehydrated versus cryopreserved amniotic membrane-derived products. There are significant differences in growth factors shown in scientific studies comparing bilayered amnion/chorion to single-layered amnion-only products as well as apparent differences in clinical outcomes when one examines studies of dehydrated and cryopreserved products.3,19,22,26 Given that, it is prudent to suggest that we cannot expect all amniotic membrane products to deliver the same healing results. While research has shown both dehydrated and cryopreserved products to be superior to standard care in randomized clinical trials, dHACM has consistently delivered healing rates in excess of 90 percent within six weeks while the cryopreserved product (Grafix) reported healing rates of 62 percent within 12 weeks.19,20,22,24
The bilayered dHACM may also accelerate time to healing. While authors have reported the median time to healing with the cryopreserved product (Grafix) to be 42 days, the median time to healing with dHACM occurred in half that time when one applies the allograft biweekly and was even more rapid (14 days) during weekly application.22,24,26
In regard to the overall number of applications, the cryopreserved product (Grafix) showed an average of six applications were necessary to achieve wound closure, while dHACM has consistently shown an average of 2.5 applications were necessary for closure.22,24,26
Looking closely at the inclusion/exclusion criteria in the cryopreserved product’s clinical study and dHACM’s clinical studies, there is an important difference in the criteria for enrollment. In the Grafix trial, researchers excluded patients healing by more than 30 percent at one week from randomization versus the more rigid criteria employed in the EpiFix clinical trials excluding patients healing more than 20 percent at 14 days. Careful examination of wound healing trajectories of the patients randomized in the respective trials suggests that the dHACM trials entered patients with wounds that were harder to heal.22,24,26
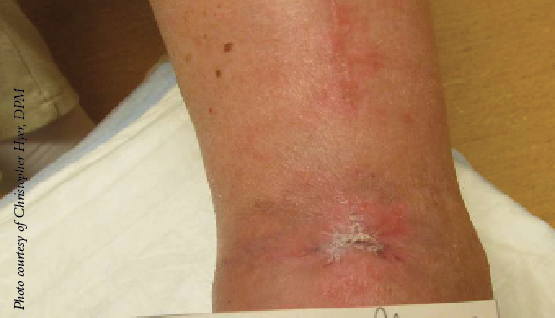 These findings suggest considering a true head-to-head trial to validate these observations and give us more information about the relative nature of different amniotic membrane products available and their ability to heal diabetic wounds.
These findings suggest considering a true head-to-head trial to validate these observations and give us more information about the relative nature of different amniotic membrane products available and their ability to heal diabetic wounds.
How Cost Effective Is Amniotic Membrane Versus Other Advanced Modalities?
Two studies in the peer-reviewed literature have compared the clinical effectiveness and cost effectiveness of dHACM versus other advanced wound products for the treatment of diabetic lower extremity ulcers.23,24 In a retrospective evaluation of chronic diabetic foot ulcers, dHACM was a more clinically effective and cost-effective treatment for diabetic foot ulcers over Apligraf (Organogenesis) and Dermagraft (Organogenesis) with high rates of rapid wound healing, a low number of grafts per healed wound, availability of multiple sized grafts and simplicity of application.23
A recent prospective, randomized, comparative effectiveness study supported these results.24 After six weeks of treatment with either dHACM or Apligraf, 95 percent of wounds treated with dHACM were healed in comparison with 45 percent of Apligraf-treated wounds. The median time to healing was significantly faster with dHACM (13 days) in comparison to Apligraf (49 days).
A Closer Look At The Research With Amniotic Membrane And Venous Ulcers
While the effectiveness of dHACM as a treatment for lower extremity ulcers in patients with diabetes appears to be established, venous leg ulcers represent the largest category of ambulatory wounds in the United States.
A multicenter, randomized controlled clinical trial of 84 patients evaluated the feasibility and efficacy of using dHACM in addition to multilayer compression therapy as a treatment for venous leg ulcers.25 Fifty-three patients received the dHACM allograft and 31 patients in the control group received multilayer compression without dHACM. The primary study outcome was the proportion of wounds achieving 40 percent closure at four weeks. At four weeks, 62 percent in the dHACM group and 32 percent of control patients demonstrated more than 40 percent wound closure, thus demonstrating a significant advantage in the dHACM-treated group in comparison to the control group at a four-week surrogate endpoint. After four weeks, wounds treated with dHACM had reduced in size by a mean of 48.1 percent in comparison to 19 percent for control patients. This study demonstrated that venous leg ulcers treated with dHACM allograft had a significant improvement in healing at four weeks in comparison to multilayered compression alone.
In Summary
Recent advances in tissue preservation techniques have resulted in commercially available amniotic membrane products for use in the clinical setting. Available data suggest that these products promote rapid and complete healing of chronic wounds. Although there are limited data available regarding most amniotic membrane-based products, there is substantial preclinical and clinical evidence supporting the rationale and effectiveness of dehydrated human amnion/chorion membrane allograft as a treatment modality. Ongoing and future studies will further define and establish the value of amniotic membrane for the treatment of other types of wounds and regenerative medicine applications.
Chronic wounds represent a significant medical challenge for patients and clinicians, and are a financial burden on the healthcare system. The rapidly growing body of evidence suggests that the properties inherent in dHACM promote tissue regeneration and healing, recruiting the patients’ own stem cells into the wounded area. Randomized controlled trials consistently demonstrate that dHACM facilitates healing. Research has shown the superior cost effectiveness of dHACM versus other advanced wound care products to be related to higher healing rates and more rapid healing, which results in less product being required to achieve healing.23,24
The availability of multiple sized grafts, which reduces waste of graft material, also contributes to cost effectiveness. Handling characteristics, a long shelf life and no need for extensive graft preparation all reduce administrative and clinical time often related with utilization of advanced wound care products, thus further supporting the cost benefits of dHACM.
Immunologically privileged amniotic membrane exhibits anti-inflammatory properties and is a reservoir of multiple growth factors involved with tissue growth and regeneration. These properties confer remarkable therapeutic potential for wound healing, tissue repair and regenerative therapy. In clinical studies and in clinical practice, amniotic membrane, especially dHACM, is showing great promise as an effective treatment for healing chronic wounds. Further studies, including head-to-head studies within the amniotic membrane space, will help further illustrate the differences between the multitude of amniotic membrane products available, and give the clinician more information to be able to chose the best product and most cost effective product for his or her patient.
Dr. Zelen is in private practice at Foot and Ankle Associates of Southwestern Virginia. He is the Medical Director of the Professional Education and Research Institute, Inc. Dr. Zelen is a Fellow of the American College of Foot and Ankle Surgeons.
Dr. Serena is the founder and CEO of the SerenaGroup. He is the President of the American Professional Wound Care Association and the Vice President of the American College of Hyperbaric Medicine.
References
- John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am. 2003;16(1):43-65.
- Mamede AC, Carvalho MJ, Abrantes AM, et al. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447-58..
- Koob TJ, Lim JJ, Zabek N, et al. Cytokines in single layer amnion allografts compared to multi-layered amnion/chorion allografts for wound healing. J Biomed Mater Res Part B Appl Biomater. 2014 Aug 30. doi: 10.1002/jbm.b.33265. [Epub ahead of print]
- Parolini O, Solomon A, Evangelista M, et al. Human term placenta as a therapeutic agent: from the first clinical applications to future perspectives. In: Berven E (ed). Human Placenta: Structure and Development. Nova Science Publishers, Hauppauge, New York, 2010, pp. 1-48.
- Baradaran-Rafii A, Aghayan H, Arjmand B, et al. Amniotic membrane transplantation. Iran J Ophthalmic Res. 2007;2(1):58-75.
- Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980;1(8179):1153–1156.
- Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202-12.
- Lopez-Valladares MJ, Rodriguez-Ares MT, Tourino R, et al. Donor age and gestational age influence on growth factor levels in human amniotic membrane. Acta Opththalmol. 2010; 88(6): e211-e216.
- Russo A, Bonci P, Bonci P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank. 2012;13(2): 353-361.
- Koob TJ, Lim JJ, Massee M, et al. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014 Aug;102(6):1353-62.
- Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10(5):493-500.
- Koob TJ, Lim JJ, Massee M, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell. 2014 ;6:10. doi: 10.1186/2045-824X-6-10. eCollection 2014.
- Chua W. Dehydrated human amnion/chorion membrane for the treatment of full-thickness plantar burn in a diabetic patient: a case report. J Diabetic Foot Complications. 2014; 6(2):67-71.
- Tenenhaus M, Greenberg M, Potenza B. Dehydrated human amnion/chorion membrane for the treatment of severe skin and tissue loss in an preterm infant: a case report. J Wound Care. 2014 Oct;23(10):490, 492-5.
- Shah A. Using amniotic membrane allografts in the treatment of neuropathic foot ulcers. J Am Podiatr Med Assoc. 2014;104(2):198-202.
- Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 2014;11(6):711-7.
- Forbes J, Fetterolf DE. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case series. J Wound Care. 2012;21(6):290-296.
- Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24(10):299-307.
- Zelen CM, Serena TE, Denoziere G, et al. A prospective randomized comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
- Zelen C. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-351.
- Zelen C, Serena T, Fetterolf D. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long term follow-up study. Wound Medicine. 2014;4:1-4.
- Zelen C, Serena T, Snyder R. A prospective randomized comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):1-14.
- Fetterolf DE, Istwan NB, Stanziano GJ. An evaluation of healing metrics associated with commonly used advanced wound care products for the treatment of chronic diabetic foot ulcers. Manag Care. 2014;23(7):31-8.
- Zelen CM, Gould L, Serena TE, et al. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2014 Nov 26. doi: 10.1111/iwj.12395. [Epub ahead of print]
- Serena TE, Carter MJ, Le LT, et al. A multi-center randomized controlled trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multi-layered compression therapy vs. multi-layer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014; 22(6)688-93.
- Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014 Oct;11(5):554-60.
- Daniel J, Tofe R, Spencer R, Russo J; MiMedx Group, Inc. (Kennesaw, GA), assignee. Placental tissue grafts. USA patent 8,357,403. 2013.
- Daniel J; MiMedx Group, Inc. (Kennesaw, GA), assignee. Placental tissue grafts. USA patent 8,372,437. 2013.
- Daniel J, Tofe R, Spencer R, Russo J; MiMedx Group, Inc. (Kennesaw, GA), assignee. Placental tissue grafts. USA patent 8,409,626. 2013.











